| In vitro: |
| Molecules. 2014 Jun 6;19(6):7497-515. | | Effect of structure on the interactions between five natural antimicrobial compounds and phospholipids of bacterial cell membrane on model monolayers.[Pubmed: 24914896] | Monolayers composed of bacterial phospholipids were used as model membranes to study interactions of the naturally occurring phenolic compounds 2,5-Dihydroxybenzaldehyde and 2-hydroxy-5-methoxybenzaldehyde, and the plant essential oil compounds carvacrol, cinnamaldehyde, and geraniol, previously found to be active against both Gram-positive and Gram-negative pathogenic microorganisms.
METHODS AND RESULTS:
The lipid monolayers consist of 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (DPPE), 1,2-dihexa- decanoyl-sn-glycero-3-phospho-(1'-rac-glycerol) (DPPG), and 1,1',2,2'-tetratetradecanoyl cardiolipin (cardiolipin). Surface pressure-area (π-A) and surface potential-area (Δψ-A) isotherms were measured to monitor changes in the thermodynamic and physical properties of the lipid monolayers. Results of the study indicated that the five compounds modified the three lipid monolayer structures by integrating into the monolayer, forming aggregates of antimicrobial -lipid complexes, reducing the packing effectiveness of the lipids, increasing the membrane fluidity, and altering the total dipole moment in the monolayer membrane model. The interactions of the five antimicrobial compounds with bacterial phospholipids depended on both the structure of the antimicrobials and the composition of the monolayers.
CONCLUSIONS:
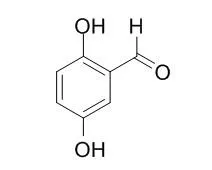
The observed experimental results provide insight into the mechanism of the molecular interactions between naturally-occurring antimicrobial compounds and phospholipids of the bacterial cell membrane that govern activities. | | Langmuir. 2012 Oct 2;28(39):14055-64. | | Diffusion-free mediator based miniature biofuel cell anode fabricated on a carbon-MEMS electrode.[Pubmed: 22946444 ] | We report on the functionalization of a micropatterned carbon electrode fabricated using the carbon-MEMS process for its use as a miniature diffusion-free glucose oxidase anode.
METHODS AND RESULTS:
Carbon-MEMS based electrodes offer precise manufacturing control on both the micro- and nanoscale and possess higher electron conductivity than redox hydrogels. However, the process involves pyrolysis in a reducing environment that renders the electrode surface less reactive and introduction of a high density of functional groups becomes challenging. Our functionalization strategy involves the electrochemical oxidation of amine linkers onto the electrode. This strategy works well with both aliphatic and aryl linkers and uses stable compounds. The anode is designed to operate through mediated electron transfer between 2,5-Dihydroxybenzaldehyde (DHB) based redox mediator and glucose oxidase enzyme. The electrode was first functionalized with ethylene diamine (EDA) to serve as a linker for the redox mediator. The redox mediator was then grafted through reductive amination, and attachment was confirmed through cyclic voltammetry. The enzyme immobilization was carried out through either adsorption or attachment, and their efficiency was compared. For enzyme attachment, the DHB attached electrode was functionalized again through electro-oxidation of aminobenzoic acid (ABA) linker. The ABA functionalization resulted in reduction of the DHB redox current, perhaps due to increased steric hindrance on the electrode surface, but the mediator function was preserved. Enzyme attachment was then carried out through a coupling reaction between the free carboxyl group on the ABA linker and the amine side chains on the enzyme. The enzyme incubation for both adsorption and attachment was done either through a dry spotting method or wet spotting method. The dry spotting method calls for the evaporation of enzyme droplet to form a thin film before sealing the electrode environment, to increase the effective concentration of the enzyme on the electrode surface during incubation. The electrodes were finally protected with a gelatin based hydrogel film. The anode half-cell was tested using cyclic voltammetry in deoxygenated phosphate buffer saline solution pH 7.4 to minimize oxygen interference and to simulate the pH environment of the body. The electrodes that yielded the highest anodic current were prepared by enzyme attachment method with dry spotting incubation.
CONCLUSIONS:
A polarization response was generated for this anodic half-cell and exhibits operation close to maximum efficiency that is limited by the mass transport of glucose to the electrode. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)