| Kinase Assay: |
| Diabetologia. 2016 Jun;59(6):1276-86. | | Bavachinin, as a novel natural pan-PPAR agonist, exhibits unique synergistic effects with synthetic PPAR-γ and PPAR-α agonists on carbohydrate and lipid metabolism in db/db and diet-induced obese mice.[Pubmed: 26983922] |
METHODS AND RESULTS:
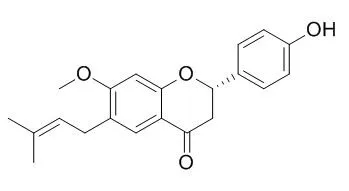
Bavachinin (BVC, a novel natural pan-PPAR agonist from the fruit of the traditional Chinese glucose-lowering herb malaytea scurfpea) exhibited glucose-lowering properties without inducing weight gain and hepatotoxicity. Importantly, BVC synergised with thiazolidinediones, which are synthetic PPAR-γ agonists, and fibrates, which are PPAR-α agonists, to induce PPAR transcriptional activity, as well as to lower glucose and triacylglycerol levels in db/db mice. We further found that BVC occupies a novel alternative binding site in addition to the canonical site of synthetic agonists of PPAR, and that the synthetic PPAR-γ agonist rosiglitazone can block BVC binding to this canonical site but not to the alternative site.
CONCLUSIONS:
This is the first report of a synergistic glucose- and lipid-lowering effect of BVC and synthetic agonists induced by unique binding with PPAR-γ or -α. This combination may improve the efficacy and decrease the toxicity of marketed drugs for use as adjunctive therapy to treat the metabolic syndrome. |
|
| Animal Research: |
| Int Immunopharmacol. 2014 Apr;19(2):399-404. | | Effect of Bavachinin and its derivatives on T cell differentiation.[Pubmed: 24508059] | Bavachinin, which can be isolated from the Chinese herb Fructus Psoraleae, has the potential as a potent anti-asthma drug. However, the extremely low water solubility of Bavachinin limits its application.
METHODS AND RESULTS:
In this study, two new derivatives of Bavachinin, i.e., compounds A and B, whose water solubility is better than that of Bavachinin, were synthesized via biotransformation. A comparative investigation was then performed on the effects of these two new derivatives, along with Bavachinin, on T cell differentiation. The results showed that they have different effects. Bavachinin and compound B inhibited green fluorescent protein (GFP) production from the T cells of IL-4-GFP-enhanced transcript (4GET) mice, whereas compound A did not. The effect was mainly attributed to the inhibition of GATA-3 protein production. Bavachinin and compound B can inhibit the production of GATA-3 mRNA, but they showed different effects on the production of T-bet mRNA. Compound B increased the production of T-bet mRNA, whereas Bavachinin did not.
CONCLUSIONS:
The results will be very useful for optimizing Bavachinin so that potent anti-allergic drugs can be developed. The structure-activity relationship of Th2 was revealed based on the difference between Bavachinin and compound B. This finding can enrich the database of preliminary drug screening from their chemical structures. |
|
| Structure Identification: |
| Bioorg Med Chem Lett. 2015 Jun 15;25(12):2579-83. | | Separation and peroxisome proliferator-activated receptor-γ agonist activity evaluation of synthetic racemic bavachinin enantiomers.[Pubmed: 25978962 ] | Bavachinin, isolated from Psoralea corylifolia seeds, has been reported to demonstrate peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist activity. However, isolated Bavachinin is actually a mixture of S and R configurations, with an enantiomeric excess value of approximately 24.3%. For further study on the structure-activity relationships of Bavachinin, investigating the PPAR-γ agonist activity of the two enantiomers is crucial.
METHODS AND RESULTS:
Considering the limited availability, racemic Bavachinin was prepared in this study using chemical synthesis. The enantiomers of racemic Bavachinin were then separated using supercritical fluid chromatography. This concise strategy yielded (S)- and (R)-Bavachinin in optical purity as high as ⩾97.5%. The PPAR-γ agonist activity of the two enantiomers was evaluated using a time-resolved fluorescence resonance energy transfer-based competitive binding assay method; IC50 values of (S)- and (R)-Bavachinin were 616.7 and 471.2 nM, respectively. The interaction between the compounds and PPAR-γ was further explored using a molecular docking method.
CONCLUSIONS:
This study suggests that (S)- and (R)-Bavachinin demonstrate similar PPAR-γ agonist activities. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)