| Description: |
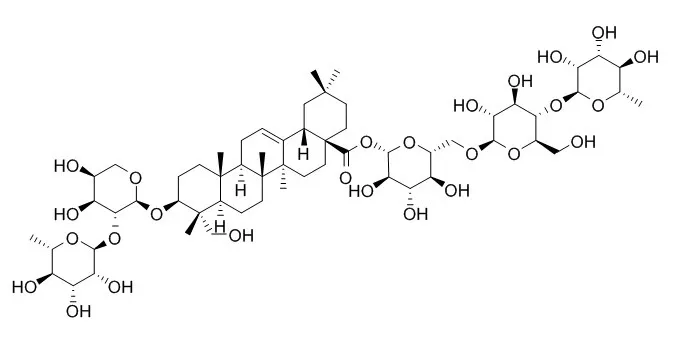
Hederacoside C is one of the active ingredients in Hedera helix leaf extract (Ivy Ex.) and AG NPP709, a new botanical drug to treat acute respiratory infection and chronic inflammatory bronchitis. Hederacoside C has antispasmodic activity. Hederacoside C is a potent competitive inhibitor for serine protease porcine pancreatic elastase, shows comparable IC 50 value is 40.6 uM; it also non-competitively inhibits hyaluronidase activity in a dose- dependent fashion, shows comparable IC 50 value is 280.4 uM. |
| In vitro: |
| Phytomedicine. 2011 Jan 15;18(2-3):214-8. | | Pre-treatment with α-hederin increases β-adrenoceptor mediated relaxation of airway smooth muscle.[Pubmed: 20637581] | Preparations of ivy leaves dry extract with secretolytic and bronchiolytic efficacy are widely used for the treatment of acute and chronic obstructive airway diseases. The mechanism by which ivy preparations improve lung functions is not fully understood.
METHODS AND RESULTS:
Here, we tested the influence of the three main saponins of ivy, α-hederin, Hederacoside C and hederagenin, on the contraction and relaxation behaviour of isolated bovine tracheal smooth muscle strips by isometric tension measurements. None of the tested compounds altered histamine or methacholine-induced contraction of the smooth muscle strips. In contrast, the isoprenaline-induced relaxation of 100μM methacholine precontracted muscle strips was significantly enhanced when pre-treated with 1μM of α-hederin for 18h. The pre-treatment with Hederacoside C or hederagenin had no effect on isoprenaline-induced relaxation.
CONCLUSIONS:
For the first time the bronchiolytic effect of α-hederin was demonstrated by isometric tension measurements using bovine tracheal smooth muscle strips. α-Hederin increases isoprenaline-induced relaxation indirectly, probably by inhibiting heterologous desensitization induced by high concentrations of muscarinic ligands like methacholine. | | J Ethnopharmacol. 2011 Apr 12;134(3):796-802. | | The effect of the whole extract of common ivy (Hedera helix) leaves and selected active substances on the motoric activity of rat isolated stomach strips.[Pubmed: 21291987] | The long tradition of using the dry extract of Hedera helix (common ivy) leaves in traditional and contemporary alternative medicine caused that many biological and pharmacological studies have been aimed at evaluating the effects of ivy. Some of the results suggest that Hedera helix extract possesses bronchodilatatory and antispasmodic activity. On the other hand, the symptoms of ivy intoxication in human and animals, as well as adverse-reactions observed during the therapy with ivy-based pharmaceuticals, indicate rather stimulant effect of the plant on smooth muscle. Thus, the aim of this study was to evaluate the effect of two main active substances extracted from the plant (α-hederin and Hederacoside C) and the whole dry extract of Hedera helix on the gut motility.
METHODS AND RESULTS:
The experiments were carried out on isolated stomach corpus and fundus strips. The tissues were isolated from rats. The experiments were performed in isotonic conditions. The results are expressed as the percent of the reaction caused by a reference contractile substance, acetylcholine. The obtained results revealed that α-hederin applied in the concentration ranged from 25 to 320μM significantly changed the spontaneous motoric activity of rat stomach smooth muscle. The observed reaction had always the same character, a contraction, and its force was concentration dependent. The second tested saponin, Hederacoside C, did not alter the motility of rat isolated stomach corpus and fundus strips when administered in the concentration up to 100 μM, however, if applied in the concentration of 350 μM it induced a remarkable concentration of smooth muscle. Eventually, the whole extract of Hedera helix in a dose containing 60 μM of Hederacoside C produced a strong contraction which strength was comparable with the reaction generated by acetylcholine.
CONCLUSIONS:
According to the results, it is very likely that α-hederin, but not Hederacoside C contributes to the contractile response of isolated stomach corpus and fundus strips to the application of Hedera helix leaves' extract. | | Planta Med. 1997 Apr;63(2):125-9. | | In vitro antispasmodic compounds of the dry extract obtained from Hedera helix.[Pubmed: 9140224] | Commercial dry extract of Hedera helix L. is used for the treatment of disorders of the respiratory tract; it is standardized towards papaverine (papaverine equivalent value, PE, activity of 1 g test substance equivalent to the activity of x mg papaverine) by in vitro antispasmodic activity on isolated guinea-pig ileum with acetylcholine as spasmogen.
METHODS AND RESULTS:
In order to determine the phytochemical basis for the antispasmodic activity, bioassay guided fractionation and subsequent isolation of phenolic compounds (flavonols, caffeoylquinic acids) and saponins (Hederacoside C, alpha-hederin, hederagenin) was carried out. Fractions and isolates obtained were investigated for antispasmodic activity and their contribution to the activity of the extract was calculated. Significant activity was found for both saponins and phenolic compounds, the PE values being approx. 55 and 49 for alpha-hederin and hederagenin, 54 and 143 for quercetin and kaempferol, and 22 for 3,5-dicaffeoylquinic acid, respectively. In view of their relative high concentration the saponins contribute most to the anti-spasmodic activity, followed by dicaffeoylquinic acids and the flavonol derivatives.
CONCLUSIONS:
The results indicate that the summed PE value of the compounds mentioned is an acceptable agreement with the PE value of the whole extract determined biologically. |
|
| In vivo: |
| Xenobiotica. 2013 Nov;43(11):985-92. | | Pharmacokinetics of hederacoside C, an active ingredient in AG NPP709, in rats.[Pubmed: 23607546 ] | 1. Hederacoside C (HDC) is one of the active ingredients in Hedera helix leaf extract (Ivy Ex.) and AG NPP709, a new botanical drug to treat acute respiratory infection and chronic inflammatory bronchitis. However, information regarding its pharmacokinetic properties remains limited.
CONCLUSIONS:
2. Here, we report the pharmacokinetics of HDC in rats after intravenous administration of HDC (3, 12.5, and 25 mg/kg) and after oral administration of HDC, Ivy Ex., and AG NPP709 (equivalent to 12.5, 25, and 50 mg/kg HDC). 3. Linear pharmacokinetics of HDC were identified upon its intravenous administration at doses of 3-25 mg/kg. Intravenous administration of HDC results in relatively slow clearance (1.46-2.08 mL/min/kg) and a small volume of distribution at steady state (138-222 mL/kg), while oral administration results in a low absolute oral bioavailability (F) of 0.118-0.250%. The extremely low F of HDC may be due to poor absorption of HDC from the gastrointestinal (GI) tract and/or its decomposition therein.
CONCLUSIONS:
4. The oral pharmacokinetics of HDC did not differ significantly among pure HDC, Ivy Ex., and AG NPP709. | | J Pharm Biomed Anal . 2016 Sep 10;129:90-95. | | An ultra-high-performance liquid chromatography-tandem mass spectrometric method for the determination of hederacoside C, a drug candidate for respiratory disorder, in rat plasma[Pubmed: 27411171] | | Abstract
Hederacoside C is a principal bioactive pharmaceutical ingredient of Hedera helix leaf extracts. H. helix extracts have long been used in folk medicine for the treatment of respiratory disorders. Currently, Hederacoside C is investigated as a promising candidate for the treatment of respiratory diseases. In this study, an accurate, sensitive, rapid, and reliable bioanalytical method was developed for the determination of Hederacoside C in rat plasma using ultra high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). For sample preparation, plasma proteins were precipitated with 0.1% acetic acid in acetonitrile. Waters UPLC BEH C18 (2.1mm I.D.×100mm, 1.7μm) column was used for chromatographic separation. A gradient elution of mobile phases consisting of 0.02% acetic acid in distilled water (solvent A) and 0.02% acetic acid in acetonitrile (solvent B) was used at a flow rate of 0.3mL/min. The multiple reaction monitoring (MRM) mode was used for mass spectrometric detection; the MRM transitions were m/z 1219.7→m/z 469.2 for Hederacoside C and m/z 1108.3→m/z 221.2 for ginsenoside Rb1 (internal standard) in the negative ionization mode. A calibration curve was constructed in the range of 10-1000ng/mL. The intra- and inter-day precision and accuracy were within 5%. The developed UPLC-MS/MS method was successfully applied in a pharmacokinetic study of Hederacoside C in rats. Hederacoside C was quickly but inadequately absorbed from the gastrointestinal tract of rats resulting in extremely low bioavailability and relatively slow clearance.
Keywords: Hedera helix; Hederacoside C; Pharmacokinetics; Plasma; UPLC–MS/MS. | | Xenobiotica . 2013 Nov;43(11):985-92. | | Pharmacokinetics of hederacoside C, an active ingredient in AG NPP709, in rats[Pubmed: 23607546] | | Abstract
1. Hederacoside C (HDC) is one of the active ingredients in Hedera helix leaf extract (Ivy Ex.) and AG NPP709, a new botanical drug to treat acute respiratory infection and chronic inflammatory bronchitis. However, information regarding its pharmacokinetic properties remains limited. 2. Here, we report the pharmacokinetics of HDC in rats after intravenous administration of HDC (3, 12.5, and 25 mg/kg) and after oral administration of HDC, Ivy Ex., and AG NPP709 (equivalent to 12.5, 25, and 50 mg/kg HDC). 3. Linear pharmacokinetics of HDC were identified upon its intravenous administration at doses of 3-25 mg/kg. Intravenous administration of HDC results in relatively slow clearance (1.46-2.08 mL/min/kg) and a small volume of distribution at steady state (138-222 mL/kg), while oral administration results in a low absolute oral bioavailability (F) of 0.118-0.250%. The extremely low F of HDC may be due to poor absorption of HDC from the gastrointestinal (GI) tract and/or its decomposition therein. 4. The oral pharmacokinetics of HDC did not differ significantly among pure HDC, Ivy Ex., and AG NPP709. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)