| In vitro: |
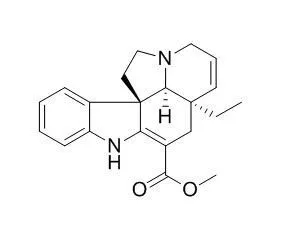
| ACS Chem Neurosci. 2015 Jun 17;6(6):879-88. | | Tabersonine Inhibits Amyloid Fibril Formation and Cytotoxicity of Aβ(1-42).[Pubmed: 25874995 ] | The misfolding and aggregation of amyloid beta (Aβ) peptides into amyloid fibrils are key events in the amyloid cascade hypothesis for the etiology of Alzheimer's disease (AD).
METHODS AND RESULTS:
Using thioflavin-T (ThT) fluorescence assay, atomic force microscopy, circular dichroism, size exclusion chromatography, surface plasmon resonance (SPR), and cytotoxicity tests, we demonstrate that Tabersonine, an ingredient extracted from the bean of Voacanga africana, disrupts Aβ(1-42) aggregation and ameliorates Aβ aggregate-induced cytotoxicity. A small amount of Tabersonine (e.g., 10 μM) can effectively inhibit the formation of Aβ(1-42) (e.g., 80 μM) fibrils or convert mature fibrils into largely innocuous amorphous aggregates. SPR results indicate that Tabersonine binds to Aβ(1-42) oligomers in a dose-dependent way. Molecular dynamics (MD) simulations further confirm that Tabersonine can bind to oligomers such as the pentamer of Aβ(1-42). Tabersonine preferentially interact with the β-sheet grooves of Aβ(1-42) containing aromatic and hydrophobic residues. The various binding sites and modes explain the diverse inhibitory effects of Tabersonine on Aβ aggregation.
CONCLUSIONS:
Given that Tabersonine is a natural product and a precursor for vincristine used in cancer chemotherapy, the biocompatibility and small size essential for permeating the blood-brain barrier make it a potential therapeutic drug candidate for treating AD. | | Phytochemistry. 2003 Sep;64(2):401-9. | | Jasmonate-induced epoxidation of tabersonine by a cytochrome P-450 in hairy root cultures of Catharanthus roseus.[Pubmed: 12943756] | Methyl jasmonate, a chemical inducer of secondary metabolism, was shown to promote Tabersonine 2 biosynthesis in hairy root cultures of Catharanthus roseus.
METHODS AND RESULTS:
Tabersonine 6,7-epoxidase activity was detected in total protein extract of jasmonate-induced hairy root cultures using labeled 14C-Tabersonine 2. This enzyme converted Tabersonine 2 to lochnericine 3 by selective epoxidation at positions 6 and 7 via a reaction dependent on NADPH and molecular oxygen. Carbon monoxide, clotrimazole, miconazole, and cytochrome C were shown to be strong inhibitors of the enzyme.
CONCLUSIONS:
The activity was found in microsomes, indicating that Tabersonine 6,7-epoxidase was a cytochrome P-450-dependent monooxygenase. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)