| Structure Identification: |
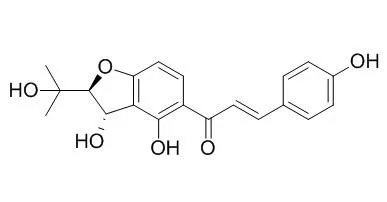
| J Org Chem. 2005 Mar 4;70(5):1761-70. | | Synthesis of 2,3-dihydro-3-hydroxy-2-hydroxylalkylbenzofurans from epoxy aldehydes. One-step syntheses of brosimacutin G, vaginidiol, vaginol, smyrindiol, xanthoarnol, and Avicenol A. Biomimetic syntheses of angelicin and psoralen.[Pubmed: 15730299] |
METHODS AND RESULTS:
We have developed two practical one-step syntheses of 2,3-dihydro-3-hydroxy-2-hydroxyalkylbenzofurans from readily available optically pure alpha,beta-epoxy aldehydes. Electron-deficient resorcinols react with epoxy aldehydes using either Cs2CO3 in DMF or KOH/CaCl2 in MeOH to give adducts 13, 16, 18, 20, 21, and Brosimacutin G (6t). Grignard reagents prepared by low-temperature halogen-metal exchange of acetoxy iodocoumarins 35d and 40 and acetoxy bromonaphthalene 41 add to epoxy aldehyde (S)-26 to complete the first syntheses of vaginidiol (7c), vaginol (7t), smyrindiol (8c), xanthoarnol (8t), and avicenol A (9t).
CONCLUSIONS:
Acid-catalyzed fragmentation of vaginidiol or vaginol provides angelicin, while that of smyrindiol or xanthoarnol affords psoralen.
In both cases, the trans isomers fragment only twice as fast as the cis isomers, possibly through the intermediacy of a common benzylic cation. This may have implications for the biosynthesis of angelicin and psoralen. | | J Nat Prod. 2002 Dec;65(12):1843-7. | | Brosimacutins A-I, nine new flavonoids from Brosimum acutifolium.[Pubmed: 12502325 ] |
METHODS AND RESULTS:
Nine new flavonoids, brosimacutins A, Brosimacutin B, Brosimacutin C, Brosimacutin D, Brosimacutin E, Brosimacutin F, Brosimacutin G, Brosimacutin H, Brosimacutin I (1-9), and four known flavonoids were isolated from the bark of Brosimum acutifolium, a Brazilian folk medicine ("Mururé").Their structures were elucidated by spectroscopic methods, including 2D NMR.
CONCLUSIONS:
Brosimacutins A-I possess differentially functionalized isoprene units at C-8. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)