| In vitro: |
| Zhongguo Zhong Yao Za Zhi. 2014 Nov;39(22):4389-93. | | Spectrum-effect relationship of Moutan cortex against lipopolysaccharide-induced acute lung injury.[Pubmed: 25850273] | This research is to study the relationship between HPLC fingerprints of Moutan Cortex, Paeoniae Radix Rubra and Paeoniae Radix Alba and their activity on lipopolysaccharide-induced acute lung injury. HPLC fingerprints of each extract of Moutan Cortex,Paeoniae Radix Rubra and Paeoniae Radix Alba were established by an optimized HPLC-MS method.
METHODS AND RESULTS:
The activities of all samples against protein and tumor necrosis a factor were tested by the model of lipopolysaccharide-induced acute lung injury. The possible relationship between HPLC-MS fingerprints and the activitieswere deduced by the Partial least squares regression analysis method. Samples were analyzed by HPLC-MS/MS to identify the major peaks. The results showed that each sample had some effect on acute lung injury. Four components with a lager contribution rate of efficacy were calculated by the research of spectrum-effect relationship.
CONCLUSIONS:
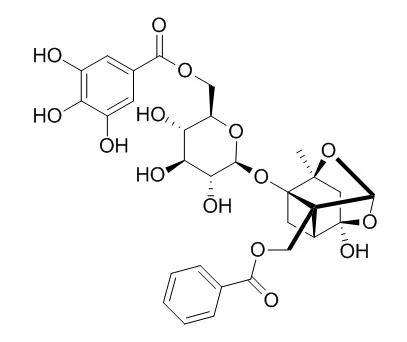
Moutan Cortex exhibited good activity on acute lung injury, and gallic acid, paeoniflorin, Galloylpaeoniflorin and paeonol were the main effective components. | | J Nat Prod. 2014 Jan 24;77(1):42-8. | | Anticomplement monoterpenoid glucosides from the root bark of Paeonia suffruticosa.[Pubmed: 24377852 ] | Six new (1-6) and 19 known monoterpenoid glucosides were isolated from the root bark of Paeonia suffruticosa.
METHODS AND RESULTS:
The monoterpenoid glucosides 1, 2, 7, 10-19, and 22 exhibited anticomplement effects with CH50 and AP50 values ranging from 0.14 to 2.67 mM and 0.25 to 3.67 mM, respectively. In a mechanistic study, suffrupaeoniflorin A (1) interacted with C1q, C3, C5, and C9, while Galloylpaeoniflorin (12) and galloyloxypaeoniflorin (19) acted on C1q, C3, and C5 components in the complement activation cascade. | | Pharmazie. 2010 Aug;65(8):624-8. | | Platelet anti-aggregatory and blood anti-coagulant effects of compounds isolated from Paeonia lactiflora and Paeonia suffruticosa.[Pubmed: 20824965] | The roots of two Paeoniaceae family members have long been used as traditional medicines in Korea, China, and Japan. Dry roots of Paeonia lactiflora and dry root bark of P. suffruticosa are used under the traditional names of Paeoniae Radix and Moutan Cortex, respectively.
METHODS AND RESULTS:
Both Paeoniae Radix and Moutan Cortex have been used as remedies for cardiovascular diseases, for improving blood circulation, or for other uses. It was postulated that both plants may contain common active constituents that contribute to inhibiting blood coagulation and/or platelet aggregation. Eighteen compounds, which have been reported to be present in both plant medicines, were evaluated for their effects on platelet aggregation and blood coagulation. Paeonol (5), paeoniflorin (9), benzoylpaeoniflorin (11), and benzoyloxypaeoniflorin (12) were found to be the major common active constituents and they would collectively contribute to improving blood circulation through their inhibitory effects on both platelet aggregation and blood coagulation. In addition, methylgallate (4), (+)-catechin (7), paeoniflorigenone (8), Galloylpaeoniflorin (13), and daucosterol (16) may also take part in improving blood circulation by inhibiting ether platelet aggregation and/or blood coagulation. | | Evid Based Complement Alternat Med . 2019 Mar 3;2019:6150357. | | The Screening Research of NF- κ B Inhibitors from Moutan Cortex Based on Bioactivity-Integrated UPLC-Q/TOF-MS[Pubmed: 30941197] | | Abstract
Inflammation is a common and important pathological process, and nuclear factor-κB (NF-κB) is a key mediator of it. Moutan Cortex (MC), the dried root cortex of Paeonia suffruticosa Andr., is widely used as a remedy for the treatment of inflammatory diseases in Asian region. However, there are few studies on the systematic identification of NF-κB inhibitors of MC. In this study, the effect of inhibiting NF-κB activation of MC was assessed at the cellular level using a tumor necrosis factor-α (TNF-α) induced inflammatory model. Subsequently, ultra-performance liquid chromatography-quadrupole/time of flight-mass spectrometry (UPLC-Q/TOF-MS) combined with biological activity assay was established to screen and identify potential anti-inflammatory ingredients in MC. The results revealed that MC significantly inhibited the activation of NF-κB. Seven potential NF-κB inhibitors were screened from MC, including oxypaeoniflorin, paeoniflorin, Galloylpaeoniflorin, benzoyloxypaeoniflorin, mudanpioside C, gallic acid, and paeonol. Among them, the NF-κB inhibitor activity of Galloylpaeoniflorin, benzoyloxypaeoniflorin, and mudanpioside C is first reported here. In conclusion, the anti-inflammatory activity of MC was associated with the seven components mentioned above. And the bioactivity-integrated UPLC-Q/TOF which contains both chemical and bioactive details is suitable for screening active ingredients from natural medicines. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)