| Kinase Assay: |
| Br J Pharmacol. 1994 Dec;113(4):1464-70. | | Antimuscarinic action of liriodenine, isolated from Fissistigma glaucescens, in canine tracheal smooth muscle.[Pubmed: 7889303 ] |
METHODS AND RESULTS:
1. The antimuscarinic properties of Liriodenine, isolated from Fissistigma glaucescens, were compared with methoctramine (cardioselective M2 antagonist) and 4-diphenylacetoxy-N-methylpiperidine (4-DAMP, smooth muscle selective M3 antagonist) by radioligand binding tests, functional tests and measurements of second messenger generation in canine cultured tracheal smooth muscle cells. 2. Liriodenine, pirenzepine, methoctramine and 4-DAMP displaced [3H]-N-methyl scopolamine ([3H]-NMS) binding in a concentration-dependent manner with Ki values of 2.2 +/- 0.4 x 10(-6), 3.3 +/- 0.7 x 10(-7), 8.9 +/- 2.3 x 10(-8) and 2.3 +/- 0.6 x 10(-9) M, respectively. The curves for competitive inhibition of [3H]-NMS with Liriodenine, methoctramine and 4-DAMP were best fitted according to a two site model of binding, but pirenzepine was best fitted according to a model with one site. 3. Liriodenine and 4-DAMP displayed a high affinity for blocking tracheal contraction (pKB = 5.9 and 9.1, respectively) and inositol phosphate formation (pKB = 6.0 and 8.9, respectively), but a low affinity for antagonism of cyclic AMP inhibition (pKB = 4.7 and 7.8, respectively). 4. Methoctramine blocked cyclic AMP inhibition with a high affinity (pKB = 7.4), but it antagonized tracheal contraction and inositol phosphate formation with a low affinity (pKB = 6.1 and 6.0, respectively).
CONCLUSIONS:
5. In conclusion, both M2 and M3 muscarinic receptor subtypes coexist in canine tracheal smooth muscle and are coupled to the inhibition of cyclic AMP formation and phosphoinositide breakdown, respectively. The antimuscarinic characteristics of Liriodenine are similar to those of 4-DAMP. It may act as a selective M3 receptor antagonist in canine tracheal smooth muscle. |
|
| Cell Research: |
| Drug Des Devel Ther. 2015 Mar 10;9:1437-48. | | Liriodenine, an aporphine alkaloid from Enicosanthellum pulchrum, inhibits proliferation of human ovarian cancer cells through induction of apoptosis via the mitochondrial signaling pathway and blocking cell cycle progression.[Pubmed: 25792804] | Liriodenine, an isoquinoline alkaloid, was examined as a potential anticancer agent, particularly in ovarian cancer.
METHODS AND RESULTS:
Liriodenine was isolated by preparative high-performance liquid chromatography. The result showed that Liriodenine inhibits proliferation of CAOV-3 cells at 37.3 μM after 24 hours of exposure. Changes in cell morphology were detected by the presence of cell membrane blebbing, chromatin condensation, and formation of apoptotic bodies. Early apoptosis was observed by Annexin V-fluorescein isothiocyanate bound to the cell membrane as early as 24 hours. Liriodenine activated the intrinsic pathway by induction of caspase-3 and caspase-9. Involvement of the intrinsic pathway in the mitochondria could be seen, with a significant increase in mitochondrial permeability and cytochrome c release, whereas the mitochondrial membrane potential was decreased. DNA fragmentation occurred at 72 hours upon exposure to Liriodenine. The presence of DNA fragmentation indicates the CAOV-3 cells undergo late apoptosis or final stage of apoptosis. Confirmation of apoptosis at the protein level showed overexpression of Bax and suppression of Bcl-2 and survivin. Liriodenine inhibits progression of the CAOV-3 cell cycle in S phase.
CONCLUSIONS:
These findings indicate that Liriodenine could be considered as a promising anticancer agent. |
|
| Animal Research: |
| Br J Pharmacol. 1996 Aug;118(7):1571-83. | | Electrophysiological mechanisms for antiarrhythmic efficacy and positive inotropy of liriodenine, a natural aporphine alkaloid from Fissistigma glaucescens.[Pubmed: 8842417] | 1. The antiarrhythmic potential and electromechanical effects of Liriodenine, an aporphine alkaloid isolated from the plant, Fissistigma glaucescens, were examined.
METHODS AND RESULTS:
2. In the Langendorff perfused (with constant pressure) rat heart, at a concentration of 0.3 to 3 microM, Liriodenine was able to convert a polymorphic ventricular tachyrhythmia induced by the ischaemia-reperfusion (EC50 = 0.3 microM). 3. In isolated atrial and ventricular muscle, Liriodenine increased the contractile force and slowed the spontaneous beating of the right atrium. 4. The Liriodenine-induced positive inotropy was markedly attenuated by a transient outward K+ channel blocker, 4-aminopyridine (4-AP) but was not significantly affected by prazosin, propranolol, verapamil or carbachol. 5. In rat isolated ventricular myocytes, Liriodenine prolonged action potential duration and decreased the maximal upstroke velocity of phase 0 depolarization (Vmax) and resting membrane potential in a concentration-dependent manner. The action potential amplitude was not significantly changed. 6. Whole-cell voltage clamp study revealed that Liriodenine blocked the Na+ channel (INa) concentration-dependently (IC50 = 0.7 microM) and caused a leftward shift of its steady-state inactivation curve. However, its recovery rate from the inactivated state was not affected. The L-type Ca2+ currents (Ica) were also decreased, but to a lesser degree (IC50 = 2.5 microM, maximal inhibition = 35%). 7. Liriodenine inhibited the 4-AP-sensitive transient outward current (Ito) (IC50 = 2.8 microM) and moderately accelerated its rate of decay. The block of Ito was not associated with changes in the voltage-dependence of the steady-state inactivation curve or in the process of recovery from inactivation of the current. Liriodenine also reduced the amplitude of a slowly inactivating, steady-state outward current (Iss) (IC50 = 1.9 microM). These effects were consistent with its prolonging effect on action potential duration. The inwardly rectifying background K+ current (IK1), was also decreased but to a less degree. 8. Compared to quinidine, Liriodenine exerted a stronger degree of block on INa, comparable degree of block on IK1, and lesser extent of block on ICa and Ito.
CONCLUSIONS:
9. It is concluded that, through inhibition of Na+ and the Ito channel, Liriodenine can suppress ventricular arrhythmias induced by myocardial ischaemia reperfusion. The positive inotropic effect can be explained by inhibition of the Ito channel and the subsequent prolongation of action potential duration. These results provide a satisfactory therapeutic potential for the treatment of cardiac arrhythmias. |
|
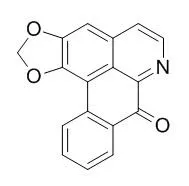
| Structure Identification: |
| J Pharm Sci. 1980 Oct;69(10):1180-3. | | Antibacterial and antifungal activity of liriodenine and related oxoaporphine alkaloids.[Pubmed: 7420287] | Liriodenine was evaluated for its antibacterial and antifungal activity against several microorganisms. Other related oxoaporphine alkaloids also were evaluated.
METHODS AND RESULTS:
Attempts to prepare oxoaporphine alkaloids from N-acetylnoraporphines were unsuccessful, but an unexpected phenanthrene alkaloid was obtained. A novel N-demethylation reaction was noted when oxogaucine methiodide and Liriodenine methiodide were treated with alumina. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)