| Description: |
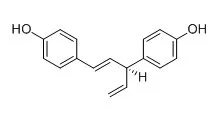
Hinokiresinol is a novel inhibitor of LTB4 binding to the human neutrophils, it has antiallergic effect, it inhibits IgE-induced mouse passive cutaneous anaphylaxis reaction. Hinokiresinol (trans-hinokiresinol) and nyasol (cis-hinokiresinol) are estrogen agonists, they possess appreciable estrogen receptor binding activity, they can stimulate the proliferation of estrogen- dependent T47D breast cancer cells, and their stimulatory effects could be blocked by an estrogen antagonist. They have similar free radical scavenging and anti-inflammatory activities, they also have anti-ischemic effects, only trans-hinokiresinol can significantly decrease neuronal injury in cultured cortical neurons exposed to oxygen-glucose deprivation followed by re-oxygenation.
|
| In vitro: |
| Chem Pharm Bull (Tokyo). 2000 Mar;48(3):389-92. | | Stereochemistry of cis- and trans-hinokiresinol and their estrogen-like activity.[Pubmed: 10726863] | Naturally occurring phenylpropanoids, hinokiresinol (trans-Hinokiresinol) and nyasol (cis-hinokiresinol) were found to possess appreciable estrogen receptor binding activity.
METHODS AND RESULTS:
Strong differences in activity were observed between the geometrical isomers and enantiomers. Among these, (3S)-cis-hinokiresinol displayed the highest activity, one order of magnitude greater than the activity of genistein. Furthermore, cis- and trans-Hinokiresinol stimulated the proliferation of estrogen-dependent T47D breast cancer cells, and their stimulatory effects were blocked by an estrogen antagonist, indicating that the compounds are estrogen agonists. In addition, the absolute configuration of C-3 in (+)-cis-hinokiresinol has been assigned as S by comparison with the circular dichroism spectra of the hydrogenated products prepared from cis and trans ((3S)-trans-Hinokiresinol: previously assigned) isomers.
CONCLUSIONS:
These results incidentally provide us with an unambiguous answer to contradictory reports regarding the assignment of the full stereochemisry of cis- and trans-Hinokiresinol that have existed in the literature for more than two decades. | | Neuropharmacology. 2013 Apr;67:465-75. | | Differential anti-ischemic efficacy and therapeutic time window of trans- and cis-hinokiresinols: stereo-specific antioxidant and anti-inflammatory activities.[Pubmed: 23287539] | During cerebral ischemia, neurons are injured by various mechanisms including excitotoxicity, oxidative stress, and inflammatory responses. Thus, pharmacological manipulation of multiple cytotoxic pathways has been pursued for the treatment of ischemic injury. Cis-hinokiresinol, a naturally occurring phenylpropanoid, was previously reported to possess anti-oxidant, anti-inflammatory and estrogen-like activities.
METHODS AND RESULTS:
In the present study, we investigated anti-ischemic effects of trans- and cis-hinokiresinols using in vitro as well as in vivo experimental models. The ORAC and DPPH assays showed that two isomers had similar free radical scavenging activities. However, only trans-Hinokiresinol significantly decreased neuronal injury in cultured cortical neurons exposed to oxygen-glucose deprivation (75 min) followed by re-oxygenation (9 h). The differential neuroprotective effect could be due to the stereo-specific augmentation of Cu/Zn-SOD activity by trans-Hinokiresinol, when compared with cis-hinokiresinol. Similarly, in rats subjected to transient middle cerebral artery occlusion (1.5 h) followed by 24-h reperfusion, pre-ischemic treatment with trans-Hinokiresinol, but not with cis-isomer, reduced cerebral infarct volume. Interestingly, however, post-ischemic treatment with both hinokiresinols (2 and 7 h after onset of ischemia) significantly reduced cerebral infarct. When administered after onset of ischemia, trans-Hinokiresinol, but not its cis-isomer reduced nitrotyrosine immunoreactivity in ischemic regions. In contrast, both hinokiresinols suppressed neutrophil infiltration and IL-1β release to a similar extent.
METHODS AND RESULTS:
The observed differential anti-oxidant, but comparable anti-inflammatory, activities may explain the stereo-specific anti-ischemic activities and different therapeutic time windows of the hinokiresinols examined.
More detailed delineation of the anti-ischemic mechanism(s) of hinokiresinols may provide a better strategy for development of efficacious regimens for cerebral ischemic stroke. | | Phytomedicine. 2007 Oct;14(10):675-80. | | Effects of Chamaecyparis formosensis Matasumura extractives on lipopolysaccharide-induced release of nitric oxide.[Pubmed: 17291735] |
METHODS AND RESULTS:
Bioactivity-guided chromatographic fractionation and metabolite profiling coupled with spectroscopic analyses, including (1)H-NMR, (13)C-NMR analyses, identified six compounds: vanillin (1), 4-hydroxybenzaldehyde (2), trans-Hinokiresinol (3), taiwanin E (4), 4alpha-hydroxyeudesm- 11-en-12-al (5), savinin (6). All of these six compounds were the first identified and reported from this tree species. Compounds (1), (3) and (5) demonstrated significant NO inhibition effect through reduction of NO production in activated RAW 264.7 cells due to the suppression of iNOS gene expression: compounds that can selectively inhibit undesirable expression of iNOS are important as they may serve as potential cancer chemopreventatives.
CONCLUSIONS:
This study suggests that C. formosensis may have potential for use as a natural resource for human health care. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)