| In vitro: |
| Int J Mol Sci. 2015 Nov 10;16(11):26850-70. | | Augmenting the Activity of Monoterpenoid Phenols against Fungal Pathogens Using 2-Hydroxy-4-methoxybenzaldehyde that Target Cell Wall Integrity.[Pubmed: 26569223] | Disruption of cell wall integrity system should be an effective strategy for control of fungal pathogens.
METHODS AND RESULTS:
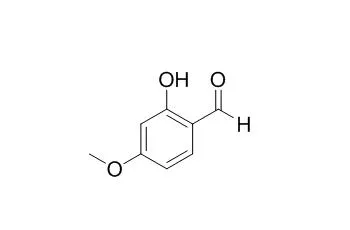
To augment the cell wall disruption efficacy of monoterpenoid phenols (carvacrol, thymol), antimycotic potency of benzaldehyde derivatives that can serve as chemosensitizing agents were evaluated against strains of Saccharomyces cerevisiae wild type (WT), slt2Δ and bck1Δ (mutants of the mitogen-activated protein kinase (MAPK) and MAPK kinase kinase, respectively, in the cell wall integrity pathway). Among fourteen compounds investigated, slt2Δ and bck1Δ showed higher susceptibility to nine benzaldehydes, compared to WT. Differential antimycotic activity of screened compounds indicated "structure-activity relationship" for targeting the cell wall integrity, where 2-Hydroxy-4-methoxybenzaldehyde (2H4M) exhibited the highest antimycotic potency. The efficacy of 2H4M as an effective chemosensitizer to monoterpenoid phenols (viz., 2H4M + carvacrol or thymol) was assessed in yeasts or filamentous fungi (Aspergillus, Penicillium) according to European Committee on Antimicrobial Susceptibility Testing or Clinical Laboratory Standards Institute M38-A protocols, respectively. Synergistic chemosensitization greatly lowers minimum inhibitory or fungicidal concentrations of the co-administered compounds. 2H4M also overcame the tolerance of two MAPK mutants (sakAΔ, mpkCΔ) of Aspergillus fumigatus to fludioxonil (phenylpyrrole fungicide).
CONCLUSIONS:
Collectively, 2H4M possesses chemosensitizing capability to magnify the efficacy of monoterpenoid phenols, which improves target-based (viz., cell wall disruption) antifungal intervention. | | Indian J Exp Biol. 2006 Oct;44(10):832-7. | | Antioxidant property of Decalepis hamiltonii Wight & Arn.[Pubmed: 17131914] | Aromatic edible root of D. hamiltonii was subjected to the extraction of the antioxidant rich fraction.
METHODS AND RESULTS:
Different parts of root namely whole tuber, peel, tuber without peel and medullary portion were extracted with dichloromethane (European Patent No. W02005063272). The extract was found to contain flavor compound 2-Hydroxy-4-methoxybenzaldehyde (2H4MB), which was identified by TLC and GC. Medullary portion was found to be rich in 2H4MB, (73.73 mg g(-1) dry tissue) followed by peel, containing 68.34 mg g(-1) 2H4MB. Different concentration of dichloromethane extracts were subjected for antioxidant assay by DPPH (1,1 dihydroxy 2-picryl hydrazyl) method, this has shown 44, 46.7% radical scavenging activity in case of medullary, peel extracts and 67.3% in case of pure 2-Hydroxy-4-methoxybenzaldehyde at 100 ppm concentration, whereas ascorbic acid used as standard showed 94.3% activity. In beta-carotene linoleate model system (b-CLAMS) 43.46 and 45.7% antioxidant activity was observed in medullary and peel extracts at 100 ppm concentrations respectively, whereas standard 2-Hydroxy-4-methoxybenzaldehyde exhibited 69.64% at 100 ppm and BHA (butylated hydroxyl anisole) 90.1% activity also at 100-ppm level. Similarly hydroxyl radical scavenging activity was found to be 48.36, 46.86, 48.26 and 73.60% in whole tuber, medullary, peel and standard 2-hydroxy-4-methoxy benzaldehyde respectively at 100 ppm levels. This is the first report on the antioxidant activity of D. hamiltonii. Results have shown that 2H4MB is one of the major constituents responsible for antioxidant activity.
CONCLUSIONS:
Hence the extract of D. hamiltonii can be utilized for the production of antioxidant rich fractions required for various health benefits. |
|
| In vivo: |
| Eur J Med Chem. 2016 May 23;114:209-19. | | Molecular modeling and snake venom phospholipase A2 inhibition by phenolic compounds: Structure-activity relationship.[Pubmed: 26986086 ] | In our earlier study, we have reported that a phenolic compound 2-Hydroxy-4-methoxybenzaldehyde from Janakia arayalpatra root extract was active against Viper and Cobra envenomations. Based on the structure of this natural product, libraries of synthetic structurally variant phenolic compounds were studied through molecular docking on the venom protein.
METHODS AND RESULTS:
To validate the activity of eight selected compounds, we have tested them in in vivo and in vitro models. The compound 21 (2-hydroxy-3-methoxy benzaldehyde), 22 (2-Hydroxy-4-methoxybenzaldehyde) and 35 (2-hydroxy-3-methoxybenzylalcohol) were found to be active against venom-induced pathophysiological changes. The compounds 20, 15 and 35 displayed maximum anti-hemorrhagic, anti-lethal and PLA2 inhibitory activity respectively.
CONCLUSIONS:
In terms of SAR, the presence of a formyl group in conjunction with a phenolic group was seen as a significant contributor towards increasing the antivenom activity. The above observations confirmed the anti-venom activity of the phenolic compounds which needs to be further investigated for the development of new anti-snake venom leads. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)