| In vitro: |
| Front Microbiol. 2017 Sep 6;8:1642. | | Antibacterial Activity of Endophytic Actinomycetes Isolated from the Medicinal Plant Vochysia divergens (Pantanal, Brazil).[Pubmed: 28932210] |
Endophytic actinomycetes from medicinal plants produce a wide diversity of secondary metabolites (SM). However, to date, the knowledge about endophytes from Brazil remains scarce. Thus, we analyzed the antimicrobial potential of 10 actinomycetes isolated from the medicinal plant Vochysia divergens located in the Pantanal sul-mato-grossense, an unexplored wetland in Brazil.
METHODS AND RESULTS:
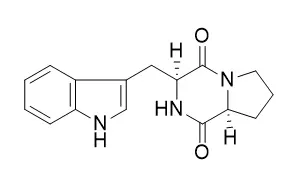
Strains were classified as belonging to the Aeromicrobium, Actinomadura, Microbacterium, Microbispora, Micrococcus, Sphaerisporangium, Streptomyces, and Williamsia genera, through morphological and 16S rRNA phylogenetic analyzes. A susceptibility analysis demonstrated that the strains were largely resistant to the antibiotics oxacillin and nalidixic acid. Additionally, different culture media (SG and R5A), and temperatures (28 and 36°C) were evaluated to select the best culture conditions to produce the active SM. All conditions were analyzed for active metabolites, and the best antibacterial activity was observed from metabolites produced with SG medium at 36°C. The LGMB491 (close related to Aeromicrobium ponti) extract showed the highest activity against methicillin-resistant Staphylococcus aureus (MRSA), with a MIC of 0.04 mg/mL, and it was selected for SM identification. Strain LGMB491 produced 1-acetyl-β-carboline (1), indole-3-carbaldehyde (2), 3-(hydroxyacetyl)-indole (4), Brevianamide F (5), and cyclo-(L-Pro-L-Phe) (6) as major compounds with antibacterial activity.
CONCLUSIONS:
In this study, we add to the knowledge about the endophytic community from the medicinal plant V. divergens and report the isolation of rare actinomycetes that produce highly active metabolites. | | J Org Chem. 2015 Aug 21;80(16):8046-54. | | Beyond the Diketopiperazine Family with Alternatively Bridged Brevianamide F Analogues.[Pubmed: 26193166 ] |
METHODS AND RESULTS:
A method for the preparation of 3,5-bridged piperazin-2-ones from a tryptophan-proline-based diketopiperazine is described using diphosgene to induce the ring closure. Density functional theory calculations were conducted to study the mechanism of this C-C bond formation. Several derivatives of the thus obtained α-chloroamine were synthesized by substitution of the chlorine atom using a range of O-, N-, S-, and C-nucleophiles.
CONCLUSIONS:
This novel class of Brevianamide F analogues possess interesting breast cancer resistance protein inhibitory activity. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)