| Structure Identification: |
| Food Chem. 2015 Jan 15;167:290-8. | | Fractionation and identification of minor and aroma-active constituents in Kangra orthodox black tea.[Pubmed: 25148991] | The aroma constituents of Kangra orthodox black tea were isolated by simultaneous distillation extraction (SDE), supercritical fluid extraction and beverage method.
METHODS AND RESULTS:
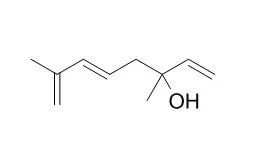
The aroma-active compounds were identified using gas chromatography-olfactometry-mass spectrometry. Geraniol, linalool, (Z/E)-linalool oxides, (E)-2-hexenal, phytol, β-ionone, Hotrienol, methylpyrazine and methyl salicylate were major volatile constituents in all the extracts. Minor volatile compounds in all the extracts were 2-ethyl-5-methylpyrazine, ethylpyrazine, 2-6,10,14-trimethyl-2-pentadecanone, acetylfuran, hexanoic acid, dihydroactinidiolide and (E/Z)-2,6-nonadienal. The concentrated SDE extract was fractionated into acidic, basic, water-soluble and neutral fractions. The neutral fraction was further chromatographed on a packed silica gel column eluted with pentane and diethyl ether to separate minor compounds. The aroma-active compounds identified using gas chromatography-olfactometry-mass spectrometry were 2-amylfuran, (E/Z)-2,6-nonadienal, 1-pentanol, epoxylinalool, (Z)-jasmone, 2-acetylpyrrole, farnesyl acetone, geranyl acetone, cadinol, cubenol and dihydroactinidiolide.
CONCLUSIONS:
AEDA studies showed 2-hexenal, 3-hexenol, ethylpyrazine, (Z/E)-linalool oxides, linalool, (E/Z)-2,6-nonadienal, geraniol, phenylethanol, β-ionone, Hotrienol and dihydroactinidiolide to be odour active components. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)