| In vitro: |
| Phytochemistry,1976,15(10):1485-7. | | The isopentenyl isoflavone luteone as a pre-infectional antifungal agent in the genus Lupinus[Reference: WebLink] | The prenylated isoflavone Luteone has been isolated from healthy leaves of Lupinus albus and 11 other lupin species.
METHODS AND RESULTS:
Evidence is presented that this compound occurs as a leaf surface constituent. In vitro tests indicate that Luteone and a second unidentified isoflavone frorn L. albus possess antifungal activity sufficient to support their proposed role as pre-infectional resistance factors.
CONCLUSIONS:
No evidence was obtained to suggest that phytoalexins were produced by the fungus-infected leaves of L. albus. | | J Ethnopharmacol . 2016 Jun 5;185:171-81. | | Antimicrobial and antioxidant activity and chemical characterisation of Erythrina stricta Roxb. (Fabaceae)[Pubmed: 26969405] | | Abstract
Ethnopharmacological relevance: The bark of Erythrina stricta Roxb. (Fabaceae) has been used in Indian indigenous systems as a remedy for rheumatism, stomach-ache, asthma, dysentery, contact dermatitis, eczema and skin infections. However, there have been limited phytochemical or biological studies on the bark of E. stricta and there are no studies that align with its traditional medicinal uses.
Aim of the study: The aim of this study was to assess the antimicrobial and antioxidant activity of the stem bark of E. stricta to support its topical use in the treatment of contact dermatitis, eczema and skin infections and to isolate and identify any bioactive compounds.
Materials and methods: MTT microdilution and disc diffusion assays were used to determine the antimicrobial activities of n-hexane, dichloromethane, ethyl acetate, methanol and water extracts of the bark of E. stricta. Column and preparative thin layer chromatography were used for the purification of the dichloromethane extract. The structures of the compounds isolated were elucidated by extensive 1D and 2D NMR spectroscopic techniques and comparison with published data. The antioxidant activities of the extracts were determined by DPPH free radical scavenging and FRAP assays and the antioxidant activity of the pure compounds by dot-blot and DPPH staining methods.
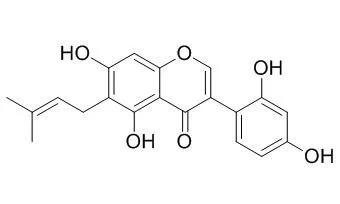
Results: The dichloromethane, ethyl acetate, and n-hexane extracts showed the most significant activity with MIC values of 7.8μg/mL, 125μg/mL, and 125μg/mL against a sensitive strain of Staphylococcus aureus. The dichloromethane and ethyl acetate extracts also showed significant activity against Candida albicans with MIC values of 125μg/mL and 1mg/mL respectively. GC-MS analysis of the n-hexane extract showed the presence of the antibacterial and antifungal compounds β-caryophyllene, caryophyllene oxide, α-selinene, β-selinene, selin-11-en-4-α-ol, α-copaene and δ-cadenine. Phytochemical studies of the dichloromethane extract led to the isolation of the novel compound erynone (1), together with six known compounds; wighteone (2), alpinum isoflavone (3), Luteone (4), obovatin (5), erythrinassinate B (6) and isovanillin (7). Luteone (4) exhibited the most significant antibacterial activity with minimum inhibitory quantity (MIQ) values of 1.88μg, 1.88μg and 3.75μg, respectively, against sensitive (MSSA) and resistant strains (MRSA and MDRSA) of S. aureus using a TLC bioautography assay. Erynone (1) exhibited the greatest DPPH free radical scavenging activity.
Conclusions: Seven compounds, including a new chromanone, were isolated from the antimicrobial dichloromethane extract of the stem bark of E. stricta. Six of the seven compounds showed antibacterial and/or antioxidant activities. These findings provide support for the customary (traditional and contemporary) use of E. stricta bark for the treatment of skin and wound infections.
Keywords: Alpinum Isoflavone (PubChem CID: 54901393), Isovanillin (PubChem ID: 12127); Antimicrobial; Antioxidant; Chemical constituents; Erythrina stricta; Isoflavanone; Luteone (PubChem CID: 5281797); Obovatin (PubChem CID: 13940733); Wighteone (PubChem ID: 5281814). |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)