| Kinase Assay: |
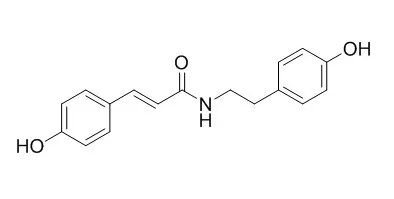
| Bioscience Biotechnology & Biochemistry, 2014, 61(7):1138-1141. | | Isolation and Activity of N-p-Coumaroyltyramine, an α-Glucosidase Inhibitor in Welsh Onion (Allium fistulosum)[Reference: WebLink] | | A phenolic amide, N-p-trans-Coumaroyltyramine(1), was isolated as an α-glucosidase inhibitor from methanol extracts of Welsh onion (Allium fistulosum). The inhibitory activity of 1 against a yeast enzyme was as high as Ki 8.4 × 10617 m. From a structure-activity relationship study of 1 and its related compounds, the occurrence of α-glucosidase inhibitory activity required a p-coumaramide structure, with an amide hydrogen and alkyl or aralkyl substituent on the amide part. |
|
| Structure Identification: |
| Nat Prod Commun. 2011 Feb;6(2):227-9. | | Amides from the stem of Capsicum annuum.[Pubmed: 21425680] |
METHODS AND RESULTS:
7'-(4'-hydroxyphenyl)-N-[(4-methoxyphenyl)ethyl]propenamide (1), 7'-(3',4'-dihydroxyphenyl)-N-[(4-methoxyphenyl)ethyl]propenamide (2), N-p-trans-Coumaroyltyramine (3), N-trans-caffeoyltyramine (4), beta-sitostenone (5), ferulic acid (6), hydroferulic acid (7), 5-hydroxy-3,4-dimethoxycinnamic acid (8), veratic acid (9), vanillic acid (10), isovanillic acid (11), syringic acid (12), (+)-syringaresinol (13), and pheophorbide a (14) were isolated from the stems of Capsicum annuum (Solanaceae). Among them, 1 is a new amide compound.
CONCLUSIONS:
The structures of these compounds were characterized and identified by spectral analyses. | | J. Phys. Chem. B, 2010, 114(8):3005-12. | | Interaction Studies of Coumaroyltyramine with Human Serum Albumin and Its Biological Importance[Pubmed: 20136105] | N-trans-p-coumaroyltyramine (N-p-trans-Coumaroyltyramine, CT) isolated from Physalis minima is a phenolic substance exhibiting many pharmacological activities like potent inhibition of acetyl cholinesterase, cell proliferation, platelet aggregation, and also antioxidant activity.
METHODS AND RESULTS:
Here, we have studied the binding of CT with HSA at physiological pH 7.2 by using fluorescence, circular dichroism spectroscopy, mass spectrometry, and molecular docking methods. From the fluorescence emission studies, the number of binding sites and binding constant were calculated to be 2 and (4.5 +/- 0.01) x 10(5) M(-1), respectively. The free energy change was calculated as -7.6 kcal M(-1) at 25 degrees C, which indicates the hydrophobic interactions of CT with HSA and is in well agreement with the computational calculations and molecular docking studies. The changes in the secondary structure of HSA after its complexation with the ligand were studied with CD spectroscopy, which indicated that the protein became partially unfolded. Also, temperature did not affect the HSA-CT complexes.
CONCLUSIONS:
The binding of CT with HSA was detected as 2 molecules bound to HSA was determined using micro TOF-Q mass spectrometry. Further, molecular docking studies revealed that CT was binding at subdomain IIA with hydrophobic interactions and also by hydrogen-bond interactions between the hydroxyl (OH) group of carbon-16 and carbon-2 of CT and Arg222, Ala291, Val293, and Met298 of HSA, with hydrogen-bond distances of 2.488, 2.811, 2.678, and 2.586 A, respectively. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)