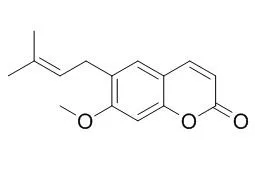
| Description: |
Suberosin exhibits anti-inflammatory, and anticoagulant activities, it also shows biting deterrent activity against Aedes aegypti, it may be useful for use as mosquito larvicidal agent. Suberosin inhibits PHA-induced PBMC proliferation, at least in part, through reduction of [Ca2+]i, ERK, NF-AT, and NF-kappaB activation, and early gene expression in PBMC including cyclins and cytokines, and arrest of cell cycle progression in the cells. |
| In vitro: |
| Br J Pharmacol. 2007 Feb;150(3):298-312. | | Suberosin inhibits proliferation of human peripheral blood mononuclear cells through the modulation of the transcription factors NF-AT and NF-kappaB.[Pubmed: 17179947] | Extracts of Plumbago zeylanica containing Suberosin exhibit anti-inflammatory activity. We purified Suberosin from such extracts and studied its effects on a set of key regulatory events in the proliferation of human peripheral blood mononuclear cells (PBMC) stimulated by phytohemagglutinin (PHA).
METHODS AND RESULTS:
Proliferation of PBMC in culture was measured by uptake of 3H-thymidine; production of cytokines and cyclins by Western blotting and RT-PCR. Transcription factors NF-AT and NF-kappaB were assayed by immunocytochemistry and EMSA. Suberosin suppressed PHA-induced PBMC proliferation and arrested cell cycle progression from the G1 transition to the S phase. Suberosin suppressed, in activated PBMC, transcripts of interleukin-2 (IL-2), interferon-gamma (IFN-gamma), and cyclins D3, E, A, and B. DNA binding activity and nuclear translocation of NF-AT and NF-kappaB induced by PHA were blocked by Suberosin. Suberosin decreased the rise in intracellular Ca2+ concentration ([Ca2+]i) in PBMC stimulated with PHA. Suberosin did not affect phosphorylation of p38 and JNK but did reduce activation of ERK in PHA-treated PBMC. Pharmacological inhibitors of NF-kappaB, NF-AT, and ERK decreased expression of mRNA for the cyclins, IL-2, and IFN-gamma and cell proliferation in PBMC activated by PHA.
CONCLUSIONS:
The inhibitory effects of Suberosin on PHA-induced PBMC proliferation, were mediated, at least in part, through reduction of [Ca2+]i, ERK, NF-AT, and NF-kappaB activation, and early gene expression in PBMC including cyclins and cytokines, and arrest of cell cycle progression in the cells. Our observations provide an explanation for the anti-inflammatory activity of P. zeylanica. | | J Nat Prod. 2011 Oct 28;74(10):2286-9. | | Structure and in vitro antiparasitic activity of constituents of Citropsis articulata root bark.[Pubmed: 21985060] | From the results of an ethnomedicinal investigation of plants from Uganda with antimalarial activity, Citropsis articulata was selected because of the antiplasmodial effect of an ethyl acetate extract of its root bark.
METHODS AND RESULTS:
Thus, from the cyclohexane, ethyl acetate, and methanol extracts, two new heterocyclic compounds, omubioside (1) and katimborine (2), were isolated in addition to five known coumarins (rutarin (3), seselin (4), Suberosin (5), demethylSuberosin (6), and haploperoside (7)), two known alkaloids (5-hydroxynoracronycine (8) and 1,5-dihydroxy-2,3-dimethoxy-10-methyl-9-acridone (9)), trigonelline (10), and the limonoid 7α-obacunyl acetate (11). The best growth inhibitors of Plasmodium falciparum were alkaloids 8 and 9, with IC50 values of 0.9 and 3.0 μg/mL. | | Rec. Nat. Prod. 10:3 (2016) 311-325. | | The identification of suberosin from prangos pabularia essential oil and its mosquito activity against aedes aegypti[Reference: WebLink] | A detailed analysis of Prangos pabularia Lindl. (Apiaceae) fruit oil was performed by gas chromatography (GC-FID) and gas chromatography-mass spectrometry (GC-MS).
METHODS AND RESULTS:
Bicyclogermacrene (21%), (Z)--ocimene (19%), -humulene (8%), -pinene (8%) and spathulenol (6%) were the main constituents of the oil. One compound with 1.8% at RI 3420 remained unidentified or tentatively identified as Suberosin from the Wiley GC-MS Library. The assumed compound, Suberosin was synthesized in two steps and its structure was confirmed by 1D NMR and GC-MS analyses. As part of our continued research to discover new chemicals for use in mosquito control agents as repellents and larvicides, Suberosin and its parent compound coumarin were investigated for the mosquito biting deterrent and larvicidal activity against Aedes aegypti. Both Suberosin and coumarin showed biting deterrent activity but the activity was lower than the positive control, DEET (N,Ndiethyl-3-methylbenzamide). In larval bioassays, Suberosin with LC50 value of 8.1 ppm was significantly more toxic than coumarin (LC50 = 49.6 ppm) at 24-h post treatment.
CONCLUSIONS:
These results indicate that Suberosin may be useful for use as mosquito larvicidal agent. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)