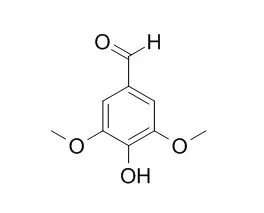
| Structure Identification: |
| Spectrochim Acta A Mol Biomol Spectrosc. 2005 Oct;61(13-14):3087-96. | | Effects of solvent, pH and beta-cyclodextrin on the photophysical properties of 4-hydroxy-3,5-dimethoxybenzaldehyde: intramolecular charge transfer associated with hydrogen bonding effect.[Pubmed: 16165057] | | The photophysical properties of 4-hydroxy-3,5-dimethoxybenzaldehyde (HDMB) in various solvents, pH and in aqueous beta-cyclodextrin (CD) have been investigated. In non-polar solvents, 4-hydroxy-3,5-dimethoxybenzaldehyde gives only one emission maxima; whereas, in polar solvents it shows a dual luminescence. The increase in Stokes shift with increase in polarity is much more for longer wavelength (LW) than for a shorter wavelength (SW) band. This behaviour indicates the formation of an intramolecular charge transfer (ICT) state through relaxation from the normal excited state. Especially in water, the ICT emission is further red shifted to 430 nm with the normal emission band at 330 nm and the relative fluorescence intensities between 330 nm and 430 nm emission bands are affected by the excitation wavelength. However, this excitation wavelength dependence is not large in aqueous beta-CD solutions. These results suggest that the ICT state in polar solvents/water is stabilized through exciplex formation by the hydrogen-bonding interaction between the carbonyl group and polar solvents/water. The ground and excited state pK(a) values for the neutral-monoanion equilibrium have been measured and discussed. 4-hydroxy-3,5-dimethoxybenzaldehyde forms a 1:1 inclusion complex with beta-CD. A mechanism is proposed to explain the inclusion process. | | J Fluoresc. 2014 May;24(3):695-707. | | Spectral and molecular modeling studies on hydroxybenzaldehydes with native and modified cyclodextrins.[Pubmed: 24310479] | | The inclusion complexation of 2-hydroxy-3-methoxybenzaldehyde (2HMB), 4-hydroxy-3-methoxybenzaldehyde (4HMB), 3,4-dimethoxybenzaldehyde (DMB) and 4-hydroxy-3,5-dimethoxybenzaldehyde (HDMB) with α-CD, β-CD, HP-α-CD and HP-β-CD were carried out by UV-Visible, steady-state and time-resolved fluorescence and PM3 methods. All the benzaldehydes shows dual fluorescence in aqueous and CD mediums and 1:1 inclusion complexes were formed with CDs. PM3 geometry optimizations results indicate that the 4-hydroxy-3,5-dimethoxybenzaldehyde /CD complex is significantly more favorable than the other complexes. The negative enthalpy changes suggest that the inclusion complexation processes are spontaneous. The geometry of the most stable complex shows that methoxy/OH group of HMBs is entrapped in the less polar CD cavities, while the aldehyde group present in the upper part of the CDs cavities. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)