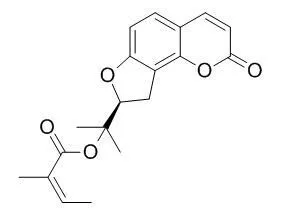
| Description: |
Columbianadin has analgesic, anti-inflammatory, calcium-channel blocking, and platelet aggregation inhibiting functions. It can effectively suppress the growth of colon cancer cells, the induction of apoptosis was correlated with the modulation of caspase-9, caspase-3, Bax, Bcl-2, RIP-3, and caspase-8, Bim and Bid. |
| Targets: |
Caspase | Bcl-2/Bax | ROS | IL Receptor | NO | Calcium Channel | RIP-3 | SOD | GPx-1 |
| In vitro: |
| Phytochemistry. 2012 Sep;81:109-16. | | Biotransformation of columbianadin by rat hepatic microsomes and inhibition of biotransformation products on NO production in RAW 264.7 cells in vitro.[Pubmed: 22784551] | Columbianadin (CBN, 1), 1-[(8S)-8,9-dihydro-2-oxo-2H-furo[2,3-h]-1-benzopyran-8-yl]-1-methylethyl-[(2Z)-2-methyl-2-butenoic acid]ester is a coumarin-type compound and one of the main bioactive constituents of the underground part of Angelica pubescens Maxim. f. biserrata Shan et Yuan.
Although numerous investigations have been undertaken to study the biological activities of Columbianadin, such as analgesic, anti-inflammatory, calcium-channel blocking, and platelet aggregation inhibiting functions, little attention has been paid to its metabolism and/or biotransformation.
METHODS AND RESULTS:
Biotransformation of Columbianadin by rat liver microsomes in vitro was studied, and thirteen biotransformation products including eight hitherto unknown compounds [columbianadiratimetins A-H (3-10)] and five known compounds [Columbianadin oxide (2), (+)-2,3-dihydro-4-hydroxy-2-(1-hydroxy-1-methylethyl)-5-benzofurancarboxaldehyde (11), oroselol (12), columbianetin (13), and vaginol (14)] were produced by liver microsomes from rats pre-treated with sodium phenobarbital. The structures of these compounds were elucidated on the basis of extensive spectroscopic analyses which included IR, UV, EIMS, HRESIMS, 1D NMR and 2D NMR, respectively.
CONCLUSIONS:
The inhibition of Columbianadin and its main biotransformation products on nitric oxide production induced by lipopolysaccharide was assayed in RAW 264.7 cells at concentrations ranging from 10 to 200 μM to evaluate the biological significance of biotransformation. | | Biomol Ther (Seoul) . 2016 May 1;24(3):320-7. | | Columbianadin Inhibits Cell Proliferation by Inducing Apoptosis and Necroptosis in HCT116 Colon Cancer Cells[Pubmed: 27098859] | | Abstract
Columbianadin (CBN), a natural coumarin from Angelica decursiva (Umbelliferae), is known to have various biological activities including anti-inflammatory and anti-cancer effects. In this study, the anti-proliferative mechanism of actions mediated by CBN was investigated in HCT-116 human colon cancer cells. CBN effectively suppressed the growth of colon cancer cells. Low concentration (up to 25 μM) of CBN induced apoptosis, and high concentration (50 μM) of CBN induced necroptosis. The induction of apoptosis by CBN was correlated with the modulation of caspase-9, caspase-3, Bax, Bcl-2, Bim and Bid, and the induction of necroptosis was related with RIP-3, and caspase-8. In addition, CBN induced the accumulation of ROS and imbalance in the intracellular antioxidant enzymes such as SOD-1, SOD-2, catalase and GPx-1. These findings demonstrate that CBN has the potential to be a candidate in the development of anti-cancer agent derived from natural products.
Keywords: Apoptosis; Colon cancer; Columbianadin; Necroptosis; Oxidative stress. | | Biomol Ther (Seoul) . 2016 May 1;24(3):320-7. | | Columbianadin Inhibits Cell Proliferation by Inducing Apoptosis and Necroptosis in HCT116 Colon Cancer Cells[Pubmed: 27098859] | | Abstract
Columbianadin (CBN), a natural coumarin from Angelica decursiva (Umbelliferae), is known to have various biological activities including anti-inflammatory and anti-cancer effects. In this study, the anti-proliferative mechanism of actions mediated by CBN was investigated in HCT-116 human colon cancer cells. CBN effectively suppressed the growth of colon cancer cells. Low concentration (up to 25 μM) of CBN induced apoptosis, and high concentration (50 μM) of CBN induced necroptosis. The induction of apoptosis by CBN was correlated with the modulation of caspase-9, caspase-3, Bax, Bcl-2, Bim and Bid, and the induction of necroptosis was related with RIP-3, and caspase-8. In addition, CBN induced the accumulation of ROS and imbalance in the intracellular antioxidant enzymes such as SOD-1, SOD-2, catalase and GPx-1. These findings demonstrate that CBN has the potential to be a candidate in the development of anti-cancer agent derived from natural products.
Keywords: Apoptosis; Colon cancer; Columbianadin; Necroptosis; Oxidative stress. |
|
| In vivo: |
| J Ethnopharmacol. 2014 Sep 11;155(2):1353-61. | | Inhibition of airway inflammation by the roots of Angelica decursiva and its constituent, columbianadin.[Pubmed: 25068578] | The roots of Angelica decursiva Fr. Et Sav (Umbelliferae) have been frequently used in traditional medicine as anti-inflammatory, antitussive, analgesic agents and expectorant, especially for treating cough, asthma, bronchitis and upper respiratory tract infections. To establish the scientific rationale for the clinical use of Angelica decursiva and to identify new agents for treating inflammatory lung disorders, pharmacological evaluation of the roots of Angelica decursiva and the isolated constituents was performed.
METHODS AND RESULTS:
In vitro study was carried out using two lung cells, lung epithelial cells (A549) and alveolar macrophages (MH-S). The inflammatory markers such as IL-6 and nitric oxide (NO) for each cell line were examined. For in vivo study, a mouse model of lipopolysaccharide (LPS)-induced acute lung injury was used and the effects on lung inflammation were established by measuring the cell numbers in bronchoalveolar lavage fluid (BALF) and by histological observation. Water and 70% ethanol extracts of the roots of Angelica decursiva showed considerable inhibitory activity against LPS-induced lung inflammation in mice following oral administration at a dose of 400 mg/kg. Five coumarin derivatives including Columbianadin, umbelliferone, umbelliferone 6-carboxylic acid, nodakenin and nodakenetin were isolated. Among the isolated compounds, Columbianadin was found to possess strong inhibitory activity against the inflammatory response of IL-1β-treated A549 cells and LPS-treated MH-S cells. Columbianadin was found to inhibit NO production by down-regulation of inducible NO synthase. Moreover, Columbianadin was also proved to possess significant inhibitory activity against LPS-induced lung inflammation following oral administration at a dose of 20-60 mg/kg.
CONCLUSIONS:
The roots of Angelica decursiva were proved to be effective in the treatment of lung inflammation. Columbianadin can be a potential new agent for treating inflammatory lung disorders. | | Biomed Chromatogr . 2016 Feb;30(2):256-62. | | Tissue distribution study of columbianadin and its active metabolite columbianetin in rats[Pubmed: 26115176] | | Abstract
Columbianadin, one of the main bioactive constituents of the roots of Angelica pubescens Maxim. f. biserrata Shan et Yuan, has been found to possess obvious pharmacological effects in previous studies. In this study, a valid and sensitive reverse-phase high-performance liquid chromatography (RP-HPLC) method was established and validated for the determination of Columbianadin (CBN) and its active metabolite columbianetin (CBT) in rat tissue samples. Sample separation was performed on an RP-HPLC column using a mobile phase of MeOH-H2 O (75:25, v/v) at a flow rate of 1.0 mL/min. The UV absorbance of the samples was measured at the wavelength 325 nm. The calibration curves for CBN were linear over the ranges of 0.5-20 μg/g for brain, testes and muscle, 1.0-10.0 μg/g for stomach and intestine, and 0.2-20.0 μg/g for heart, liver, spleen, lung and kidney. The calibration curves for CBT were linear over the ranges of 0.5-25 μg/g for stomach and intestine, and 0.1-10.0 μg/g for heart, liver, spleen, lung and kidney. The analysis method was successfully applied to a tissue distribution study of CBN and CBT after intravenous administration of CBN to rats. The results of this study indicated that CBN could be detected in all of the selected tissues after i.v. administration. CBN was distributed to rat tissues rapidly and could be metabolized to CBT in most detected tissues. Of the detected tissues, heart had the highest uptake of CBN, which suggested that heart might be one of the main target tissues of CBN. Concentrations of CBT were obviously higher in the digestive system than in other assayed tissues. The information provided by this research is very useful for gaining knowledge of the capacities of CBN and CBT to access different tissues.
Keywords: Angelica pubescens Maxim. f. biserrata; RP-HPLC; Columbianadin; columbianetin; tissue distribution. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)