| Structure Identification: |
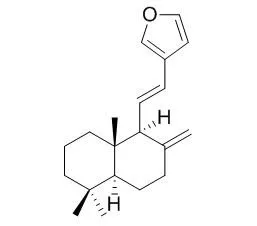
| Chem Pharm Bull (Tokyo). 2008 Mar;56(3):398-403. | | Concise syntheses of coronarin A, coronarin E, austrochaparol and pacovatinin A.[Pubmed: 18310958] | Total syntheses of (+)-coronarin A (1), (+)-Coronarin E (2), (+)-austrochaparol (3) and (+)-pacovatinin A (4) were achieved from the synthetic (+)-albicanyl acetate (6).
METHODS AND RESULTS:
Dess-Martin oxidation of (+)-albicanol (5) derived from the chemoenzymatic product (6) gave an aldehyde (7), which was subjected to Julia one-pot olefination using beta-furylmethyl-heteroaromatic sulfones (8 or 9 ) gave (+)-trans Coronarin E (2) and (+)-cis Coronarin E (12) with high cis-selectivity. The synthesis of (+)-coronarin A (1) from (+)-trans Coronarin E (2) was achiev-ed, while (+)-cis Coronarin E (12) was converted to the natural products (+)-(5S,9S,10S)-15,16-epoxy-8(17),13(16),14-labdatriene (13) and (+)-austrochaparol (3).
CONCLUSIONS:
By the asymmetric synthesis of (+)-3, the absolute structure of (+)-3 was determined to be 5S, 7R, 9R, 10S configurations. Homologation of (+)-albicanol (5) followed by allylic oxidation gave (7 alpha)-hydroxy nitrile (17), which was finally converted to the natural (+)-pacovatinin A (4) in 8 steps from (+)-albicanol (5).
|
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)