| Structure Identification: |
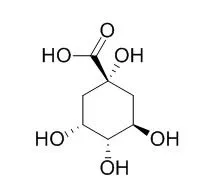
| Inorg Chem. 2013 Dec 16;52(24):13849-60. | | Heptanuclear antiferromagnetic Fe(III)-D-(-)-quinato assemblies with an S = 3/2 ground state-pH-specific synthetic chemistry, spectroscopic, structural, and magnetic susceptibility studies.[Pubmed: 24266671] | Iron is an essential metal ion with numerous roles in biological systems and advanced abiotic materials. D-(-)-Quinic acid is a cellular metal ion chelator, capable of promoting reactions with metal M(II,III) ions under pH-specific conditions.
METHODS AND RESULTS:
In an effort to comprehend the chemical reactivity of well-defined forms of Fe(III)/Fe(II) toward α-hydroxycarboxylic acids, pH-specific reactions of: (a) [Fe3O(CH3COO)6(H2O)3]·(NO3)·4H2O with D-(-)-Quinic acid in a molar ratio 1:3 at pH 2.5 and (b) Mohr's salt with D-(-)-Quinic acid in a molar ratio 1:3 at pH 7.5, respectively, led to the isolation of the first two heptanuclear Fe(III)-quinato complexes, [Fe7O3(OH)3(C7H10O6)6]·20.5H2O (1) and (NH4)[Fe7(OH)6(C7H10O6)6]·(SO4)2·18H2O (2). Compounds 1 and 2 were characterized by analytical, spectroscopic (UV-vis, FT-IR, EPR, and Mössbauer) techniques, CV, TGA-DTG, and magnetic susceptibility measurements. The X-ray structures of 1 and 2 reveal heptanuclear assemblies of six Fe(III) ions bound by six doubly deprotonated quinates and one Fe(III) ion bound by oxido- and hydroxido-bridges (1), and hydroxido-bridges (2), all in an octahedral fashion. Mössbauer spectroscopy on 1 and 2 suggests the presence of Fe(III) ions in an all-oxygen environment.
CONCLUSIONS:
EPR measurements indicate that 1 and 2 retain their structure in solution, while magnetic measurements reveal an overall antiferromagnetic behavior with a ground state S = 3/2. The collective physicochemical properties of 1 and 2 suggest that the (a) nature of the ligand, (b) precursor form of iron, | | J Org Chem. 2007 May 25;72(11):4258-61. | | Expeditious synthesis of tri- and tetrahydroxyazepanes from D-(-)-quinic acid as potent glycosidase inhibitors.[Pubmed: 17480095] | Several new stereoisomers of 3,4,6-trihydroxyazepanes and 7-hydroxymethyl-3,4,5-trihydroxyazepanes as well as known 3,4,5-trihydroxyazepanes were synthesized as potent glycosidase inhibitors from D-(-)-Quinic acid in an efficient manner.
METHODS AND RESULTS:
The key step employs dihydroxylation of protected chiral 1,4,5-cyclohex-2-enetriols under RuCl3/NaIO4/phosphate buffer (pH 7) condition, followed by reductive amino cyclization. We found the choice of an appropriate protecting group to C1-OH of chiral 1,4,5-cyclohex-2-enetriols would increase the yields of cyclization.
CONCLUSIONS:
The preliminary biological data indicate some of these azepanes possess potent inhibition against alpha-mannosidase and alpha-fucosidase. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)