| Structure Identification: |
| Molecules. 2017 Aug 14;22(8). | | Inositol Derivatives and Phenolic Compounds from the Roots of Taraxacum coreanum.[Pubmed: 28805750 ] |
METHODS AND RESULTS:
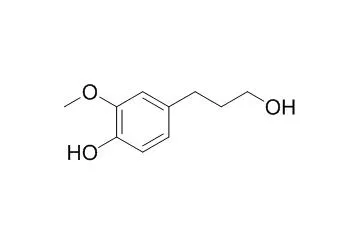
In this study, the characterization of chemical constituents and biological activity of the roots of Taraxacum coreanum (Asteraceae) was attempted. Phytochemical investigation of the roots of T. coreanum led to the isolation of two new inositol derivatives, taraxinositols A (1) and B (2), and a new phenolic compound, taraxinol (16), together with twenty known compounds including four inositol derivatives, neo-inositol-1,4-bis (4-hydroxybenzeneacetate) (3), chiro-inositol-1,5-bis(4- hydroxybenzeneacetate) (4), chiro-inositol-2,3-bis (4-hydroxybenzeneacetate) (5) and chiro-inositol- 1,2,3-tris (4-hydroxybenzeneacetate) (6), nine phenolic compounds: p-hydroxybenzaldehyde (7), vanillin (8), syringaldehyde (9), vanillic acid (10), 4-methoxyphenylacetic acid (11), 4-hydroxy- phenylacetic acid methyl ester (12), optivanin (13), isoferulic acid (14) and Dihydroconiferyl alcohol (15), four coumarins: nodakenetin (17), decursinol (18), prangol (19) and isobyakangelicin (20), and three lignans: syringaresinol-4'-O-β-d-glucoside (21), syringaresinol (22), and pinoresinol (23).
The structures of isolated compounds were determined on the basis of spectroscopic analysis.
CONCLUSIONS:
Among the isolated compounds, vanillic acid, isoferulic acid and syringaresinol showed radical scavenging activity with IC50 values ranging from 30.4 to 75.2 μM. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)