| Description: |
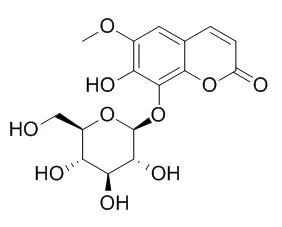
Fraxin possesses a variety of bioactivities such as anti-inflammatory, antioxidant, analgesic, antimicrobial, antiviral, immunomodulatory, anti-hyperuricemia and diuresis. Fraxin enhances urate excretion partly by inhibiting mURAT1 or mGLUT9 in kidney of hyperuricemic mice. |
| Targets: |
GLUT | mURAT1 | mOAT1 | mOCT1 |
| In vitro: |
| Exp Mol Med. 2005 Oct 31;37(5):436-46. | | Natural compounds,fraxin and chemicals structurally related to fraxin protect cells from oxidative stress.[Pubmed: 16264268] | Coumarins comprise a group of natural phenolic compounds found in a variety of plant sources. In view of the established low toxicity, relative cheapness, presence in the diet and occurrence in various herbal remedies of coumarins, it appears prudent to evaluate their properties and applications further.
METHODS AND RESULTS:
The purpose of this study is to investigate cellular protective activity of coumarin compound, Fraxin extracted from Weigela florida var. glabbra, under oxidative stress, to identify genes expressed differentially by Fraxin and to compare antioxidative effect of Fraxin with its structurally related chemicals. Of the coumarins, protective effects of Fraxin against cytotoxicity induced by H2O2 were examined in human umbilical vein endothelial cells (HUVECs). Fraxin showed free radical scavenging effect at high concentration (0.5 mM) and cell protective effect against H2O2-mediated oxidative stress. Fraxin recovered viability of HUVECs damaged by H2O2-treatment and reduced the lipid peroxidation and the internal reactive oxygen species level elevated by H2O2 treatment. Differential display reverse transcription-PCR revealed that Fraxin upregulated antiapoptotic genes (clusterin and apoptosis inhibitor 5) and tumor suppressor gene (ST13). Based on structural similarity comparing with Fraxin, seven chemicals, fraxidin methyl ether (29.4% enhancement of viability), prenyletin (26.4%), methoxsalen (20.8%), diffratic acid (19.9%), rutoside (19.1%), xanthyletin (18.4%), and kuhlmannin (18.2%), enhanced more potent cell viability in the order in comparison with Fraxin, which showed only 9.3% enhancement of cell viability.
CONCLUSIONS:
These results suggest that Fraxin and Fraxin-related chemicals protect HUVECs from oxidative stress. |
|
| In vivo: |
| J Nat Prod. 2006 May;69(5):755-7. | | Metabolic fate of fraxin administered orally to rats.[Pubmed: 16724835] |
METHODS AND RESULTS:
Naturally occurring Fraxin (1) was administered orally to rats to investigate its metabolism. Urinary metabolites were analyzed by three-dimensional HPLC, and fraxetin-7-O-sulfate (2), fraxetin-7-O-beta-glucuronide (3), fraxetin (4), 6,7,8-trihydroxycoumarin (5), and fraxidin (6) were isolated. Fraxin (1) was extensively metabolized to 4, which was partly metabolized to 5 in a rat fecal suspension after incubation for 24 h. Urinary excretion of 4 and 5 in rats administered orally with 1 was substantially reduced when the rats were treated with antibiotics to suppress their intestinal flora. Incubation of 1 with a rat liver S-9 mixture yielded 6.
CONCLUSIONS:
These results suggest that hydrolysis and demethylation of 1 are performed by intestinal microflora, while methylation occurs in the liver. | | Eur J Pharmacol. 2011 Sep;666(1-3):196-204. | | Protective effects of cortex fraxini coumarines against oxonate-induced hyperuricemia and renal dysfunction in mice.[Pubmed: 21620826] | The aim of the present study was to investigate the effects of cortex Fraxini coumarines esculetin, esculin, fraxetin and Fraxin on renal dysfunction and expression abnormality of renal organic ion transporters in hyperuricemic animals.
METHODS AND RESULTS:
Mice were orally given 250 mg/kg oxonate for seven consecutive days to induce hyperuricemia and renal dysfunction. After 1h of oxonate induction daily, animals were orally treated with esculetin, esculin, fraxetin and Fraxin at 20 and 40 mg/kg, respectively. Esculetin, esculin, fraxetin and Fraxin significantly decreased serum urate, creatinine and blood urea nitrogen levels and increased urine urate and creatinine excretion in hyperuricemic mice. Esculetin and esculin up-regulated expressions of renal organic anion transporter 1 (mOAT1), organic cation and carnitine transporters (mOCT1-2 and mOCTN1-2), but failed to affect renal glucose transporter 9 (mGLUT9) and urate transporter 1 (mURAT1) in this model. Fraxetin specifically inhibited renal mURAT1, while Fraxin extensively interacted with renal mGLUT9, mURAT1, mOAT1 and mOCT1 in hyperuricemic mice. Furthermore, esculetin, fraxetin and Fraxin increased mABCG2 mRNA expression and decreased its protein levels in renal apical membrane in hyperuricemic mice.
CONCLUSIONS:
These results indicate that esculetin and esculin have beneficial effects on hyperuricemia and renal dysfunction, resulting in restoration of mOAT1, mOCT1-2 and mOCTN1-2, and fraxetin and Fraxin enhance urate excretion partly by inhibiting mURAT1 or mGLUT9 in kidney of hyperuricemic mice. Regulation of mABCG2 by cortex Fraxini coumarines may be partly contributed to their beneficial actions. This study provides an evidence to support clinical therapeutic effects of cortex Fraxini coumarines on hyperuricemia with renal dysfunction. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)