METHODS AND RESULTS:
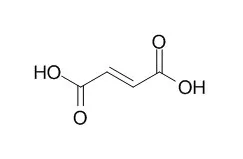
In this study, Escherichia coli was metabolically engineered for the production of Fumaric acid under aerobic condition. For the aerobic production of Fumaric acid, the iclR gene was deleted to redirect the carbon flux through the glyoxylate shunt. In addition, the fumA, fumB, and fumC genes were also deleted to enhance Fumaric acid formation. The resulting strain was able to produce 1.45 g/L of Fumaric acid from 15 g/L of glucose in flask culture. Based on in silico flux response analysis, this base strain was further engineered by plasmid-based overexpression of the native ppc gene, encoding phosphoenolpyruvate carboxylase (PPC), from the strong tac promoter, which resulted in the production of 4.09 g/L of Fumaric acid. Additionally, the arcA and ptsG genes were deleted to reinforce the oxidative TCA cycle flux, and the aspA gene was deleted to block the conversion of Fumaric acid into L-aspartic acid. Since it is desirable to avoid the use of inducer, the lacI gene was also deleted. To increase glucose uptake rate and Fumaric acid productivity, the native promoter of the galP gene was replaced with the strong trc promoter. Fed-batch culture of the final strain CWF812 allowed production of 28.2 g/L Fumaric acid in 63 h with the overall yield and productivity of 0.389 g Fumaric acid/g glucose and 0.448 g/L/h, respectively.
CONCLUSIONS:
This study demonstrates the possibility for the efficient production of Fumaric acid by metabolically engineered E. coli. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)