| In vitro: |
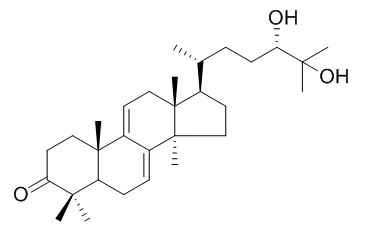
| Food Chem Toxicol. 2013 Mar;53:317-24. | | Protective effect of ganodermanondiol isolated from the Lingzhi mushroom against tert-butyl hydroperoxide-induced hepatotoxicity through Nrf2-mediated antioxidant enzymes.[Pubmed: 23266269] | Ganodermanondiol, a biologically active compound, was isolated from the Lingzhi mushroom (Ganoderma lucidum). The present study examined the protective effects of Ganodermanondiol against tert-butyl hydroperoxide (t-BHP)-induced hepatotoxicity.
METHODS AND RESULTS:
Ganodermanondiol protected human liver-derived HepG2 cells through nuclear factor-E2-related factor 2 (Nrf2) pathway-dependent heme oxygenase-1 expressions. Moreover, Ganodermanondiol increased cellular glutathione levels and the expression of the glutamine-cysteine ligase gene in a dose-dependent manner. Furthermore, Ganodermanondiol exposure enhanced the phosphorylation of adenosine monophosphate-activated protein kinase (AMPK) and its upstream kinase activators, LKB1 and Ca(2+)/calmodulin-dependent protein kinase-II (CaMKII).
CONCLUSIONS:
This study indicates that Ganodermanondiol exhibits potent cytoprotective effects on t-BHP-induced hepatotoxicity in human liver-derived HepG2 cells, presumably through Nrf2-mediated antioxidant enzymes and AMPK. | | Planta Med. 2001 Dec;67(9):811-4. | | Anticomplement activity of terpenoids from the spores of Ganoderma lucidum.[Pubmed: 11745016] | A new lanostane-type terpenoid, lucidenic acid SP1 (1), was isolated from a CHCl(3)-soluble fraction of Ganoderma lucidum spores together with four other known compounds (2 - 5). The structure of lucidenic acid SP1 was determined to be 3 beta,7 beta-dihydroxy-4,4,14 alpha-trimethyl-11,15-dioxo-5 alpha-chol-8-en-24-oic acid by spectroscopic means including 2D-NMR.
METHODS AND RESULTS:
Twelve triterpenes (1-12) isolated from G. lucidum spores were investigated in vitro for their anticomplementary activity. Compounds 1 - 5 were inactive, whereas ganoderiol F (8), Ganodermanondiol (9) and ganodermanontriol (10) showed a strong anticomplement activity against the classical pathway (CP) of the complement system with IC(50) values of 4.8, 41.7, and 17.2 microM, respectively. The potency of these triterpene alcohols (8-10) in inhibiting CP activity was improved when the number of hydroxymethyl groups on the side chain moiety is increased. On the other hand, the ganoderic acids 1-7, which contain a carboxyl group in the side chain, and lucidumols A and B (11, 12) had little activity on this system. | | Int J Mol Sci . 2016 Oct 27;17(11):1798. | | Effects of Ganodermanondiol, a New Melanogenesis Inhibitor from the Medicinal Mushroom Ganoderma lucidum[Pubmed: 27801787] | | Abstract
Ganoderma lucidum, a species of the Basidiomycetes class, has been attracting international attention owing to its wide variety of biological activities and great potential as an ingredient in skin care cosmetics including "skin-whitening" products. However, there is little information available on its inhibitory effect against tyrosinase activity. Therefore, the objectives of this study were to investigate the chemical composition of G. lucidum and its inhibitory effects on melanogenesis. We isolated the active compound from G. lucidum using ethanol extraction and ethyl acetate fractionation. In addition, we assayed its inhibitory effects on tyrosinase activity and melanin biosynthesis in B16F10 melanoma cells. In this study, we identified a bioactive compound, Ganodermanondiol, which inhibits the activity and expression of cellular tyrosinase and the expression of tyrosinase-related protein-1 (TRP-1), TRP-2, and microphthalmia-associated transcription factor (MITF), thereby decreasing melanin production. Furthermore, Ganodermanondiol also affected the mitogen-activated protein kinase (MAPK) cascade and cyclic adenosine monophosphate (cAMP)-dependent signaling pathway, which are involved in the melanogenesis of B16F10 melanoma cells. The finding that Ganodermanondiol from G. lucidum exerts an inhibitory effect on tyrosinase will contribute to the use of this mushroom in the preparation of skin care products in the future.
Keywords: B16F10 melanoma cell; Ganoderma lucidum; Ganodermanondiol; microphthalmia-associated transcription factor; tyrosinase; tyrosinase-related proteins. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)