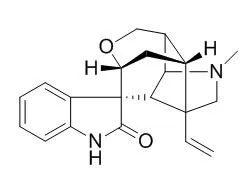
The present study investigated the anti-hyperlipidemic proprieties of a natural alkaloid, Gelsemine, in a high-fat-fed rabbit model.
METHODS AND RESULTS:
Animals were randomly divided into five groups and fed normal diet, hypercholesterolemic diet (1% cholesterol), or hypercholesterolemic diet (1% cholesterol) supplemented with Gelsemine (1, 5, or 25 mg/kg). After 60 days, serum concentrations of total cholesterol (TC), LDL-C, HDL-C, triglycerides, apolipoproteins A and B, SGOT, SGPT, glucose, and insulin were measured in all experimental groups. Hypercholesterolemic diet resulted in significantly elevated levels of TC, TG, LDL-C, SGOT, and SGPT, and reduced HDL-C compared to the normocholesterolemic diet group. Gelsemine treatment significantly improved lipid profile parameters, affected by hyperlipidemia, while having no effect on the levels of apolipoproteins, glucose, and insulin. Furthermore, Gelsemine treatment decreased hyperlipidemia-induced oxidative stress in a dose-dependent manner, as indicated by the increased activity of superoxide dismutase and catalase, and reduction in serum nitric oxide, and malondialdehyde concentrations in hyperlipidemic animals that received Gelsemine supplementation.
CONCLUSIONS:
Dietary supplementation with Gelsemine may, therefore, reverse the effect of the lipogenic diet on lipid profile and hepatic enzymes in hyperlipidemic rabbits, and protect tissues from oxidative stress, caused by high-fat diet. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)