| Structure Identification: |
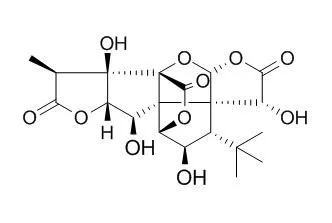
| Acta Crystallogr C. 2002 Mar;58(Pt 3):o195-8. | | Three ginkgolide hydrates from Ginkgo biloba L.: ginkgolide A monohydrate, ginkgolide C sesquihydrate and ginkgolide J dihydrate, all determined at 120 K.[Pubmed: 11870327] | A low-temperature structure of ginkgolide A monohydrate, (1R,3S,3aS,4R,6aR,7aR,7bR,8S,10aS,11aS)-3-(1,1-dimethylethyl)-hexahydro-4,7b-dihydroxy-8-methyl-9H-1,7a-epoxymethano-1H,6aH-cyclopenta[c]furo[2,3-b]furo[3',2':3,4]cyclopenta[1,2-d]furan-5,9,12(4H)-trione monohydrate, C(20)H(24)O(9) x H(2)O, obtained from Mo K alpha data, is a factor of three more precise than the previous room-temperature determination.
METHODS AND RESULTS:
A refinement of the ginkgolide A monohydrate structure with Cu K alpha data has allowed the assignment of the absolute configuration of the series of compounds. Ginkgolide C sesquihydrate, (1S,2R,3S,3aS,4R,6aR,7aR,7bR,8S,10aS,11S,11aR)-3-(1,1-dimethylethyl)-hexahydro-2,4,7b,11-tetrahydroxy-8-methyl-9H-1,7a-epoxymethano-1H,6aH-cyclopenta[c]furo[2,3-b]furo[3',2':3,4]cyclopenta[1,2-d]furan-5,9,12(4H)-trione sesquihydrate, C(20)H(24)O(11) x 1.5H(2)O, has two independent diterpene molecules, both of which exhibit intramolecular hydrogen bonding between OH groups. Ginkgolide J dihydrate, (1S,2R,3S,3aS,4R,6aR,7aR,7bR,8S,10aS,11aS)-3-(1,1-dimethylethyl)-hexahydro-2,4,7b-trihydroxy-8-methyl-9H-1,7a-epoxymethano-1H,6aH-cyclopenta[c]furo[2,3-b]furo[3',2':3,4]cyclopenta[1,2-d]furan-5,9,12(4H)-trione dihydrate, C(20)H(24)O(10) x 2H(2)O, has the same basic skeleton as the other ginkgolides, with its three OH groups having the same configurations as those in Ginkgolide C.
CONCLUSIONS:
The conformations of the six five-membered rings are quite similar across ginkgolides A-C and J, except for the A and F rings of ginkgolide A. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)