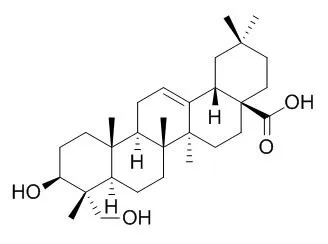
| Description: |
Hederagenin shows anti-cancer,anti-inflammatory, antidepressant-like ,anticomplementary, and antimutagenic effects, it can evoke hemolysis on the erythrocytes, and has cytotoxic on various tumor cell lines, P-388, L-1210, U-937, HL-60, SNU-5 and HepG2. Hederagenin can inhibit LPS-stimulated expression of iNOS, COX-2, and NF-κB, regulate monoamine neurotransmitters and 5-HTT mRNA expression.
|
| In vitro: |
| BMC Complement Altern Med. 2014 Oct 24;14:412. | | Hederagenin from the leaves of ivy (Hedera helix L.) induces apoptosis in human LoVo colon cells through the mitochondrial pathway.[Pubmed: 25342273] | Colorectal cancer has become one of the leading cause of cancer morbidity and mortality throughout world. Hederagenin, a derivative of oleanolic acid isolated from the leaves of ivy (Hedera helix L.), has been shown to have potential anti-tumor activity. The study was conducted to evaluate whether Hederagenin could induce apoptosis of human colon cancer LoVo cells and explore the possible mechanism.
METHODS AND RESULTS:
MTT assay was used for evaluating cell viability while Annexin V-FITC/PI assay and Hoechst 33342 nuclear stainining were used for the determination of apoptosis and mitochondrial membrane potential. DCFH-DA fluorescence staining and flow cytometry were used to measure ROS generation. Real-time PCR and western blot analysis were performed for apoptosis-related protein expressions.
MTT assay showed that Hederagenin could significantly inhibit the viability of LoVo cells in a concentration-dependent and time-dependent manner by IC50 of 1.39 μM at 24 h and 1.17 μM at 48 h. The apoptosis ratio was significantly increased to 32.46% and 81.78% by the induction of Hederagenin (1 and 2 μM) in Annexin V-FITC/PI assay. Hederagenin could also induce the nuclear changes characteristic of apoptosis by Hoechst 33342 nuclear stainining under fluorescence microscopy. DCFH-DA fluorescence staining and flow cytometry showed that Hederagenin could increase significantly ROS generation in LoVo cells. Real-time PCR showed that Hederagenin induced the up-regulation of Bax and down-regulation of Bcl-2, Bcl-xL and Survivin. Western blotting analysis showed that Hederagenin decreased the expressions of apoptosis-associated proteins Bcl-2, procaspase-9, procaspase-3, and polyADP- ribosepolymerase (PARP) were increased, while the expressions of Bax, caspase-3, caspase-9 were increased. However, there was no significant change on caspase-8.
CONCLUSIONS:
These results indicated that the disruption of mitochondrial membrane potential might contribute to the apoptosis of Hederagenin in LoVo cells. Our findings suggested that Hederagenin might be a promising therapeutic candidate for human colon cancer. | | Arch Pharm Res. 1999 Jun;22(3):317-9. | | In vitro anticomplementary activity of hederagenin saponins isolated from roots of Dipsacus asper.[Pubmed: 10403139] | Anticomplementary activity of Hederagenin and related saponins isolated from Dipsacus asper was investigated in vitro.
METHODS AND RESULTS:
HN saponin F (3) was most potent with IC50 value of 3.7x10(-5) M followed by 3-O-beta-D-glucopyranosyl-(1->3)-alpha-L-rhamnopyranosyl-(1->2)-beta-L-+ ++arabi nopyranosyl Hederagenin 28-O-beta-D-glucopyranosyl-(1->6)-beta-D-glucopyrano side (8), 3-O-beta-L-arabinopyranosyl Hederagenin 28-O-beta-D-glucopyranosyl-(1->6)-beta-D-glucopyranoside (5), dipsacus saponin A (4), and Hederagenin (1) on the classical pathway (CP) of complement system, while the saponins 3-5 did not show the inhibition of hemolysis and rather increase the hemolysis on the alternative pathway (AP).
METHODS AND RESULTS:
However, all of C-3 monodesmosides [prosapogenin CP (2), dipsacus saponin B (6), and dipsacus saponin C (7)] evoked hemolysis directly on the erythrocytes. | | Int Immunopharmacol . 2015 Dec;29(2):528-537. | | Hederagenin, a major component of Clematis mandshurica Ruprecht root, attenuates inflammatory responses in RAW 264.7 cells and in mice[Pubmed: 26481049] | | Abstract
Clematis mandshurica Ruprecht root has been used in Asia as a traditional anti-inflammatory, analgesic, and antitumor agent. Its main active component is Hederagenin, a naturally occurring triterpene, and in this study, we examined the anti-inflammatory effects of Hederagenin in lipopolysaccharide-stimulated RAW 264.7 cells using an enzyme-linked immunosorbent assay, Western blot, and RT-PCR. In addition, its effects on acute inflammation in vivo were observed using a carrageenan-induced mouse hind paw edema assay. Furthermore, the changes on the histopathology and histomorphometry of hind paw skins were examined using carrageenan-treated mice. Treatment with Hederagenin (10, 30 and 100μM) resulted in inhibited levels of protein expression of lipopolysaccharide-stimulated iNOS, COX-2, and NF-κB as well as production of NO, PGE2, TNF-α, IL-1β, and IL-6 induced by lipopolysaccharide. Consistent with these results, Hederagenin also dose-dependently reduced the lipopolysaccharide-induced mRNA levels of iNOS and COX-2, and of the above-mentioned cytokines. Interestingly, results of the carrageenan-induced mouse hind paw edema assay showed an anti-edema effect of Hederagenin. Furthermore, Hederagenin (30mg/kg) inhibited the carrageenan-induced increases in skin thicknesses, infiltrated inflammatory cells, and mast cell degranulation. These results suggest that Hederagenin may possess anti-inflammatory activities.
Keywords: Hederagenin; Histological examination; Inflammatory response; Mouse paw edema; Nuclear factor-kappa B. |
|
| In vivo: |
| Pharm Biol. 2015 Mar;53(3):368-77. | | Involvement of norepinephrine and serotonin system in antidepressant-like effects of hederagenin in the rat model of unpredictable chronic mild stress-induced depression.[Pubmed: 25471378] | Previous studies from our laboratory indicated that both acute and subchronic administration of Fructus Akebiae (FAE) [the fruit of Akebiae quinata (Thunb.) Decne, (Lardizabalaceae)] produce antidepressant-like effects in animal depressive behavior tests. FAE contains approximately 70% of Hederagenin (HG) as its main chemical component.
This study compared the antidepressant ability of FAE with that of HG in mice and further investigated the antidepressant-like effects and potential mechanisms of HG in rats subjected to unpredictable chronic mild stress (UCMS).
METHODS AND RESULTS:
Mice received FAE (50 mg/kg) and HG (20 mg/kg) once a day via intragastric administration (i.g.) for 3 weeks. The anxiolytic and antidepressant activities of FAE and HG were compared using elevated plus maze (EPM) and behavioral despair tests including tail suspension test (TST) and forced swimming test (FST), respectively. Antidepressant effects of HG (5 mg/kg) were assessed using the UCMS depressive rat model. Moreover, the levels of monoamine neurotransmitters and relevant gene expression in UCMS rats' hippocampi were determined through high-performance liquid chromatography with electrochemical detection and real-time polymerase chain reaction techniques.
The results of our preliminary screening test suggest that HG at 20 mg/kg, while not FAE at 50 mg/kg, significantly decreased the immobility in both TST and FST compared with the vehicle group when administered chronically; however, there were no significant differences observed between the HG and the FAE group. Chronic administration of HG failed to significantly reverse the altered crossing and rearing behavioral performance, time spent in the open arm and closed entries in the EPM, even if they showed an increased tendency, but HG significantly increased the percent of sucrose preference in the sucrose preference test (SPT) and decreased the immobility time in the FST. HG showed that significant increases of norepinephrine and serotonin levels and exhibited a tendency to increase the expression of 5-hydroxytryptamine (serotonin) 1A receptor mRNA, and to significantly decrease the expression of the mRNA for the serotonin transporter (5-HTT). However, there were no significant differences in the expression of the brain-derived neurotrophic factor.
CONCLUSIONS:
These findings confirm the antidepressant-like effects of HG in a behavioral despair test and UCMS rat model, which may be associated with monoamine neurotransmitters and 5-HTT mRNA expression. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)