| Description: |
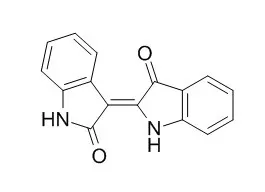
Indirubin is a potent cyclin-dependent kinases and GSK-3β inhibitor with IC50 of about 5 μM and 0.6 μM. Indirubin has anticancer, anti-inflammatory,antiviral,anti-allergic contact dermatitis effects. Each indirubin derivative acts on the DNA binding of NF-Y and represses the MDR1 gene promoter with tumor cell-type specificity.Indirubin derivatives have a potential to be used as an adjunct to antiviral therapy for the treatment of severe human H5N1 disease. |
| Targets: |
Bcl-2/Bax | Caspase | NF-kB | IkB | MAPK | Antifection | STAT | IKK | GSK-3β | IFN-γ | IL Receptor | TNF-α | CXCL10 |
| In vitro: |
| Oncol Lett. 2015 Apr;9(4):1940-1946. | | Enhancing effects of indirubin on the arsenic disulfide-induced apoptosis of human diffuse large B-cell lymphoma cells.[Pubmed: 25789073] | The aim of the present study was to investigate the Indirubin-enhanced effects of arsenic disulfide (As2S2) on the proliferation and apoptosis of diffuse large B-cell lymphoma (DLBCL) cells in order to identify an optimum combination therapy.
METHODS AND RESULTS:
The human DLBCL cells, LY1 and LY8, were treated with different concentrations of Indirubin for 24, 48 and 72 h. Next, the cells were treated with 10 μM As2S2 or a combination of 10 μM As2S2 and 20 μM Indirubin for 48 h.The DLBCL cell viability exhibited no significant changes at 24, 48 or 72 h with increasing Indirubin concentration. In addition, the apoptotic rates of the LY1 and LY8 cells demonstrated no noticeable effects at 48 h with increasing Indirubin concentration. Following treatment with the combination of Indirubin and As2S2, the inhibitory and apoptotic rates of the cells were notably increased compared with those of the As2S2-treated group. The qPCR results revealed that Indirubin alone had no enhancing effect upon the Bax/Bcl-2 mRNA expression ratio and caspase-3 mRNA expression. Western blot analysis revealed that Indirubin alone had an enhancing effect upon the Bax/Bcl-2 protein ratio and procaspase-3 protein expression. In addition, the results demonstrated that the 21-KDa Bax protein was proteolytically cleaved into an 18-KDa Bax in the DLBCL cells treated with the combination of Indirubin and As2S2. Indirubin alone did not inhibit proliferation or induce the apoptosis of the LY1 and LY8 cells. However, the combination of Indirubin and As2S2 yielded enhancing effects.
CONCLUSIONS:
Therefore, the results of the present study demonstrated that with regard to antitumor activities, As2S2 served as the principal drug, whereas Indirubin served as the adjuvant drug. The enhancing effect was due, in part, to the induction of the mitochondrial apoptotic pathway, which involves the cleavage of Bax. |
|
| In vivo: |
| J Ethnopharmacol. 2013 Jan 9;145(1):214-9. | | Indirubin, a purple 3,2- bisindole, inhibited allergic contact dermatitis via regulating T helper (Th)-mediated immune system in DNCB-induced model.[Pubmed: 23149289] | Indirubin, isolated from Indigo naturalis (Apiaceae) is a purple 3,2- bisindole and a stable isomer of indigo. Although it is known to have anti-inflammatory activities, its mechanism of action has not been elucidated.
METHODS AND RESULTS:
Seven-week-old female BALB/c mice were sensitized with 1-chloro-2,4-dinitrobenzene (DNCB) to induce skin inflammation. Hematoxylin and eosin staining was performed to assess epidermal and dermal hyperplasia, which were determined by measuring the thicknesses of the epidermis and dermis, respectively. We also evaluated serum immunoglobulin E (IgE) levels and cytokines production, such as tumor necrosis factor (TNF)-alpha, interleukin (IL)-4, 6 and Interferon (IFN)-gamma. In addition, we investigated nuclear factor (NF)-κB, IκB-α and mitogen-activated protein (MAP) kinase activities for verifying the molecular mechanism of inflammation.
Indirubin treatment suppressed skin inflammation in DNCB-exposed mice. The skin lesions were significantly thinner in the Indirubin-treated group than in untreated controls, and the hyperkeratosis disappeared. Indirubin reduced the total serum IgE level and cytokines production. In addition, it normalized NF-κB, IκB-α and MAP kinase expression.
CONCLUSIONS:
Indirubin might be a useful treatment for allergic contact dermatitis via regulating the co-expression of T helper (Th) 1 and 2 cell-mediated immune responses. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)