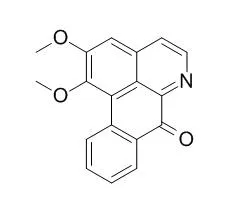
| Description: |
Lysicamine shows significant antioxidant capacity in the ORAC(FL) assay and it is active against S. epidermidis and C. dubliniensis, with MIC values in the range 12.5-100 microg mL(-1). Lysicamine has antimicrobial and anti-inflammation activity, the minimum inhibitory concentrations of the pour component (Streptococcus mutans, Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans and Candida albicans.) is less than 5mg/ml for all four microorganisms and the minimum concentration with anti-inflammation is 0.2mg/ml for both IL-8 and IL-6. Lysicamine has antileishmanial activity. It can significantly inhibit the proliferation of melanoma cells, it has antioxidative activity and chemopreventive activity in skin melanoma cells. |
| In vitro: |
| Applied Mechanics & Materials, 2011, 108:189-93. | | Lysicamine in a Lotus Leaves Extract May Be Responsible for Antibacterial and Anti-Inflammation Activity[Reference: WebLink] |
METHODS AND RESULTS:
In the course of a search for chemotherapeutic agents inhibiting suspected peridontitis bacteria, extracted and purified substances from lotus leaf were identified by antimicrobial and Anti-inflammation activity tests with use of the broth micro-dilution methods on 96-microwell plate and ELISA assay. The antimicrobial activity of extracts was tested against four microorganisms, namely: Streptococcus mutans , Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans and Candida albicans. The Anti-inflammation activity of extracts was tested for inhibition of inflammatory cytokines IL-8 and IL-6 in macrophage BF24. And one pure component was subjected to spectroscopic analysis using UV, IR, EMI-MS, EIMS, 1H-NMR, 13C-NMR, 1H-COSY and HMQC. Our data showed that the minimum inhibitory concentrations of the pour component was less than 5mg/ml for all four microorganisms and the minimum concentration with anti-inflammation was 0.2mg/ml for both IL-8 and IL-6. The component was determined to be Lysicamine.
CONCLUSIONS:
Thus, we conclude that Lysicamine extracted from lotus leaves may be a potential antibacterial and Anti-inflammation agent for oral infection. | | Molecules, 2014, 19(4):4234-45. | | Antioxidant and anticancer constituents from the leaves of Liriodendron tulipifera.[Pubmed: 24705566 ] |
METHODS AND RESULTS:
Sixteen compounds were extracted and purified from the leaves of Liriodendron tulipifera. These compounds include aporphines, oxoaporphine, coumarin, sesquiterpene lactone, benzenoids, cyclitol and steroids. (+)-Norstephalagine (2) (an aporphine) and scopoletin (8) (a coumarin) were isolated from Liriodendron tulipifera leaves from the first time. The identified compounds were screened for their antiradical scavenging, metal chelating and ferric reducing power activities. The results have showed that these compounds have antioxidative activity. The study has also examined the chemopreventive property of the isolated compounds against human melanoma cells A375. The results shown that (-)-anonaine (1), (-)-liridinine (3), (+)-lirinidine (6), Lysicamine (7) and epitulipinolide diepoxide (9) significantly inhibited the proliferation of melanoma cells.
CONCLUSIONS:
These results revealed that these compounds have antioxidative activity and chemopreventive activity in skin melanoma cells. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)