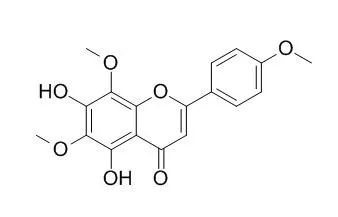
| Structure Identification: |
| Acta Pharmacol Sin. 2002 Jul;23(7):667-72. | | Structure-activity relationship of natural flavonoids in hydroxyl radical-scavenging effects.[Pubmed: 12100765] | To study the relationship between the structure and hydroxyl radical (*OH)-scavenging activity of twelve natural flavonoids.
METHODS AND RESULTS:
The hydroxyl radical-generating chemiluminescence system with ascorbate-CuSO4-yeast-H2O2 was used to determine the hydroxyl radical-scavenging activity of twelve natural flavonoids.
Guercetin, heliosin, hyperoside, kaempferol, baicalin, corylifolin, Lysionotin, matteucinol, corylifolinin, and genistein could effectively scavenge. OH and inhibit the chemiluminescence of the system. The IC50 values (95 % confidence limits) of the flavonoids were 12.1 (9.9-14.5) g/L, 15.8(14.0-19.2) g/L, 19.5 (16.8-27.4) g/L, 20.1 (13.6-29.0) g/L, 34.6 (28.4-43.4) g/L, 66.8 (63.2-74.4) g/L, 187 (147-235) g/L, 211 (165-284) g/L, 262 (190-346) g/L, and 708 (498-994) g/L, respectively; whereas nobilelin and corylifolin-Ac could not scavenge *OH.
CONCLUSIONS:
(1) Phenolic hydroxyls in flavonoids were the main active groups capable of scavenging *OH; (2) Hydroxyl groups in ring B and A were important *OH-scavenging active groups; (3) The ortho-dihydroxyl groups in ring A and/or B could greatly enhance the *OH-scavenging activity of the rings; (4) Comparing the IC50 values of guercetin, heliosin, hyperoside, baicalin, Lysionotin, and matteucinol, it was suggested that the hydroxyl groups on 3',4' position of ring B possessed high *OH-scavenging activity and the scavenging activity of hydroxyl groups in ring B was higher than that of hydroxyl groups in ring A. The hydroxyl group or glucoside on 3 position of ring C of the above mentioned 6 flavonoids was also related to the. OH-scavenging ability. (5) The structural types of flavonoids themselves could influence their *OH-scavenging activity. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)