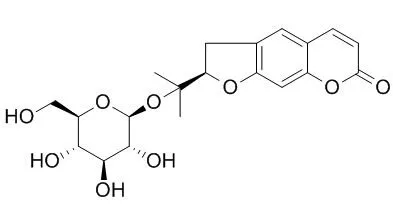
| Description: |
Nodakenin acts as an AChE inhibitor that inhibits AChE activity in a dosedependent manner with an IC50 value of 84.7 μM. Nodakenin possesses neuroprotective, anti-allergic, antiaggregatory, antibacterial, and memory -enhancing effects. Nodakenin down-regulates the expression of the proinflammatory iNOS, COX-2, TNF-α, IL-6, and IL-1β genes in macrophages by interfering with the activation of TRAF6, thus preventing NF-κB activation.
|
| Targets: |
NOS | COX | TNF-α | IL Receptor | p65 | NF-kB | IkB | NO | PGE | Antifection | IKK | TRAF6 | AChR |
| In vitro: |
| J Pharmacol Exp Ther. 2012 Sep;342(3):654-64. | | Nodakenin suppresses lipopolysaccharide-induced inflammatory responses in macrophage cells by inhibiting tumor necrosis factor receptor-associated factor 6 and nuclear factor-κB pathways and protects mice from lethal endotoxin shock.[Pubmed: 22637723] | Nodakenin, a coumarin isolated from the roots of Angelicae gigas, has been reported to possess neuroprotective, antiaggregatory, antibacterial, and memory-enhancing effects.
METHODS AND RESULTS:
In the present study, we investigated the anti-inflammatory effects of Nodakenin by examining its in vitro inhibitory effects on inducible nitric-oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and proinflammatory cytokines in lipopolysaccharide (LPS)-induced RAW 264.7 macrophages and mouse peritoneal macrophages and its in vivo effects on LPS-induced septic shock in mice. Our results indicate that Nodakenin concentration-dependently inhibits iNOS and COX-2 at the protein, mRNA, and promoter binding levels, and these inhibitions cause attendant decreases in the production of nitric oxide (NO) and prostaglandin E₂ (PGE₂). Furthermore, we found that Nodakenin inhibits the production and mRNA expression of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-1β induced by LPS. Molecular data revealed that Nodakenin suppressed the transcriptional activity and translocation of nuclear factor-κB (NF-κB) by inhibiting inhibitory κB-α degradation and IκB kinase-α/β phosphorylation. In addition, Nodakenin was found to significantly inhibit the LPS-induced binding of transforming growth factor-β-activated kinase 1 to tumor necrosis factor receptor-associated factor 6 (TRAF6) by reducing TRAF6 ubiquitination. Pretreatment with Nodakenin reduced the serum levels of NO, PGE₂, and proinflammatory cytokines and increased the survival rate of mice with LPS-induced endotoxemia.
CONCLUSIONS:
Taken together, our data suggest that Nodakenin down-regulates the expression of the proinflammatory iNOS, COX-2, TNF-α, IL-6, and IL-1β genes in macrophages by interfering with the activation of TRAF6, thus preventing NF-κB activation. |
|
| In vivo: |
| Immunopharmacol Immunotoxicol. 2014 Oct;36(5):341-8. | | The effects of nodakenin on airway inflammation, hyper-responsiveness and remodeling in a murine model of allergic asthma.[Pubmed: 25090633] | Nodakenin is a major coumarin glucoside in the root of Peucedanum decursivum Maxim, a commonly used traditional Chinese medicine for the treatment of asthma and chronic bronchitis for thousands of years.
In this work, the anti-asthma potential of Nodakenin was studied by investigation of its effect to suppress airway inflammation, hyper-responsiveness and remodeling in a murine model of chronic asthma.
METHODS AND RESULTS:
BALB/c mice sensitized to ovalbumin (OVA) were challenged with aerosolized OVA for 8 weeks, orally administered with Nodakenin at doses of 5, 10 and 20 mg/kg before each OVA challenge.
Compared with the model group, Nodakenin treatment markedly inhibited airway inflammation, hyper-responsiveness and remodeling, showing improvement in subepithelial fibrosis, smooth muscle hypertrophy, and goblet cell hyperplasia, and decreased levels of interleukin (IL)-4, IL-5, IL-13 and matrix metalloproteinase-2/-9 in bronchoalveolar lavage fluid, and the level of OVA-specific IgE in serum. In addition, the NF-κB DNA-binding activity in lung tissues was also reduced by Nodakenin treatment.
CONCLUSIONS:
These data indicated that Nodakenin might mitigate the development of chronic experimental allergic asthma. | | Life Sci. 2007 May 1;80(21):1944-50. | | Nodakenin, a coumarin compound, ameliorates scopolamine-induced memory disruption in mice.[Pubmed: 17382968 ] | Nodakenin is a coumarin compound initially isolated from the roots of Angelica gigas.
METHODS AND RESULTS:
In the present study, we investigated the effects of Nodakenin on learning and memory impairments induced by scopolamine (1 mg/kg, i.p.) using the passive avoidance test, the Y-maze test, and the Morris water maze test in mice. Nodakenin (10 mg/kg, p.o.) administration significantly reversed scopolamine-induced cognitive impairments in the passive avoidance test and the Y-maze test (P<0.05), and also reduced escape latency during training in the Morris water maze test (P<0.05). Moreover, swimming times and distances within the target zone of the Morris water maze were greater in the Nodakenin-treated group than in the scopolamine-treated group (P<0.05). In an in vitro study, Nodakenin was found to inhibit acetylcholinesterase activity in a dose-dependent manner (IC(50)=84.7 microM). In addition, Nodakenin was also found to inhibit acetylcholinesterase activity for 6 h in an ex-vivo study.
CONCLUSIONS:
These results suggest that Nodakenin may be a useful for the treatment of cognitive impairment, and that its beneficial effects are mediated, in part, via the enhancement of cholinergic signaling. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)