| In vitro: |
| Nat Prod Res. 2013;27(18):1677-81. | | Cytotoxic activity of Guettarda pohliana Müll. Arg. (Rubiaceae).[Pubmed: 23387288] |
METHODS AND RESULTS:
The cytotoxic activity of crude extracts and their fractions from leaves and roots of G. pohliana was assessed against nine human cancer cell lines: melanoma (UACC-62), breast (MCF-7), breast expressing the multidrug resistance phenotype (NCI-ADR), lung (NCI-460), prostate (PCO-3), kidney (786-0), ovarian (OVCAR), colon (HT-29) and leukaemia (K-562). The hexane fraction from leaves (HL) and ethyl acetate (EAR), chloroform (CR) and hydromethanolic (HMR) fractions from roots were the most active fractions against K-562 with GI₅₀ values being lower than 1 μg mL⁻1. Also, CR and HMR fractions were active against UACC-62 cell line in the same order of magnitude.
CONCLUSIONS:
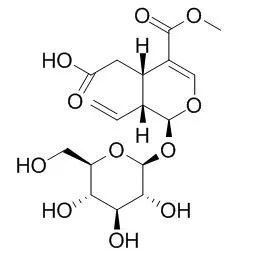
The phytochemical study of the CR fraction allowed identifying the known iridoids Secoxyloganin, sweroside and loganin. | | Food Chem. 2013 May 1;138(1):327-33. | | Screening and identification of the antibacterial bioactive compounds from Lonicera japonica Thunb. leaves.[Pubmed: 23265495] | Our aim was to screen for antibacterial bioactive compounds from Lonicera japonica leaves. Staphylococcus aureus and Escherichia coli were used as the indicator bacteria.
Bacteriostatic assay-guided extraction and stepwise partitioning of the samples yielded five compounds of interest.
METHODS AND RESULTS:
Antimicrobial activities of the compounds were determined using a disk diffusion assay. Extracts, fractions, and compounds from L. japonica leaves possessed considerable antibacterial activities against the tested bacterial strains and the most active fraction was attributed to J3B2, which primarily contained 3,5-di-O-caffeoylquinic acid and 4,5-di-O-caffeoylquinic acid. Meanwhile, five bacteriostatic constituents were isolated (3-O-caffeoylquinic acid, Secoxyloganin, luteoloside, 3,5-di-O-caffeoylquinic acid and 4,5-di-O-caffeoylquinic acid), among which, Secoxyloganin was isolated for the first time from leaves. The antibacterial activity of the compounds was in the order of 3,5-bis-O-caffeoyl quinic acid, 4,5-bis-O-caffeoylquinic acid, luteoloside>3-O-caffeoylquinic acid>Secoxyloganin.
CONCLUSIONS:
Our results suggested that the phenolic compounds might significantly contribute to antibacterial activity and were the most responsible for the bacteriostatic activity of L. japonica leaves. | | Chemistry & Industry of Forest Products, 2005, 25(3):29-32. | | CHEMICAL CONSTITUENTS IN FLOWER BUDS OF LONICERA JAPONICA THUNB.[Reference: WebLink] | Active constituents were isolated from the flower buds of Lonicera japonica Thunb. They were extracted with 70 % alcohol, then fractioned successively with petroleum ether, ethyl acetate and n-butanol.
METHODS AND RESULTS:
The ethyl acetate part was repeatedly chromatographed on silica gel column and purified with ODS column. Nine compounds, namely loganin(1),sweroside(2),7-epi-vogeloside(3),7-epi-loganin(4),Secoxyloganin(5),caffeic acid(6),p-hydroxybenzoic acid(7), β-sitosterol(8) and daucosterol (9) were obtained and their structures were deduced by comparison of their physico-chemical properties and spectral data with those of reference data.
CONCLUSIONS:
Compounds 4,5,6,7 were found for the first time in the flower buds of L. japonica Thunb., and compounds 1,4,5 were found to possess hepato- protective effect. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)