| In vivo: |
| Biol Psychiatry. 2014 Jun 1;75(11):840-6. | | Yohimbine enhancement of exposure therapy for social anxiety disorder: a randomized controlled trial.[Pubmed: 24237691] | Preclinical and clinical trials suggest that Yohimbine may augment extinction learning without significant side effects. However, previous clinical trials have only examined adults with specific phobias. Yohimbine has not yet been investigated in the augmentation of exposure therapy for other anxiety disorders.
METHODS AND RESULTS:
Adults (n = 40) with a DSM-IV diagnosis of social anxiety disorder were randomized to placebo or Yohimbine HCl (10.8 mg) 1 hour before each of four exposure sessions. Outcome measures were collected at baseline, each treatment session, posttreatment, and 1-month follow-up.
Yohimbine was well tolerated. Yohimbine augmentation, relative to placebo augmentation, resulted in faster improvement and better outcomes on self-report measures of social anxiety disorder severity (Liebowitz Social Anxiety Scale, d = .53) and depressed mood severity (Beck Depression Inventory, d = .37) but not on the clinician-rated measures (Clinical Global Impressions-Severity Scale, d = .09; Clinical Global Impressions-Improvement Scale, d = .25). Between-group differences on the Liebowitz Social Anxiety Scale were moderated by the level of fear reported at the end of an exposure exercise (end fear), such that the advantage of Yohimbine over placebo was only evident among patients who reported low end fear.
CONCLUSIONS:
The results provide moderate support for Yohimbine as a therapeutic augmentation strategy for exposure therapy in social anxiety disorder, one that may be especially effective when coupled with successful exposure experiences. Beneficial effects for Yohimbine were readily evident for self-report measures but not for clinician-rated outcomes of social anxiety severity and improvement. | | Psychopharmacology (Berl). 2007 Dec;195(3):345-55. | | The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats.[Pubmed: 17705061 ] | Yohimbine is an alpha-2 adrenoreceptor antagonist that provokes stress- and anxiety-like responses in both humans and laboratory animals. In rats, Yohimbine increases operant alcohol self-administration and reinstates alcohol seeking. In this study, we assess whether these effects of Yohimbine are attenuated by systemic injections of the corticotrotropin-releasing factor 1 (CRF1) receptor antagonist antalarmin.
METHODS AND RESULTS:
In Exp. 1, we trained rats to lever press for alcohol solutions (12% w/v, 1 h/day) over several weeks; during training, the response requirement was increased from a fixed-ratio-1 (FR-1) to a fixed-ratio-3 (FR-3) reinforcement schedule. We then tested the effect of antalarmin (10 or 20 mg/kg) on Yohimbine (1.25 mg/kg)-induced increases in operant alcohol self-administration (FR-3 reinforcement schedule). Subsequently, we assessed the effect of antalarmin on Yohimbine-induced increases in plasma corticosterone levels in the previously self-administering rats. In Exp. 2, we trained the rats to self-administer alcohol as in Exp. 1, and after extinction of the alcohol-reinforced lever responding over 13 days, we tested antalarmin's effect on Yohimbine-induced reinstatement of alcohol seeking.
Yohimbine increased operant alcohol self-administration and reinstated alcohol seeking after extinction. These effects of Yohimbine were attenuated by antalarmin. Antalarmin injections in the absence of Yohimbine had no effect on either operant alcohol self-administration or extinction responding. Antalarmin had no effect on Yohimbine-induced corticosterone release in alcohol-experienced rats.
CONCLUSIONS:
These results suggest that extrahypothalamic CRF1 receptors are involved in the effect of Yohimbine on operant alcohol self-administration and on relapse to alcohol seeking and support the notion that CRF1 receptor antagonists should be considered in alcohol addiction treatment. |
|


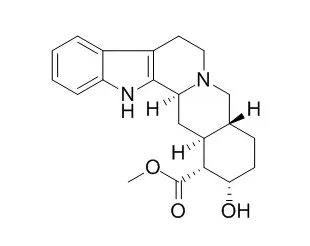



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)