| In vitro: |
| Parasitol Res. 2012 Feb;110(2):539-44. | | Antimalarial drug interactions of compounds isolated from Kigelia africana (Bignoniaceae) and their synergism with artemether, against the multidrug-resistant W2mef Plasmodium falciparum strain.[Pubmed: 21814840] | For decades, drug resistance has been the major obstacle in the fight against malaria, and the search for new drugs together with the combination therapy constitutes the major approach in responding to this situation.
METHODS AND RESULTS:
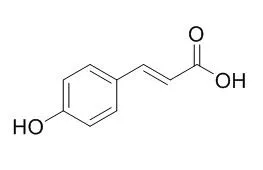
The present study aims at assessing the in vitro antimalarial activity of four compounds isolated from Kigelia africana stem bark (atranorin - KAE1, specicoside - KAE7, 2β,3β,19α-trihydroxy-urs-12-20-en-28-oic acid - KAE3, and p-Hydroxy-cinnamic acid - KAE10) and their drug interactions among themselves and their combination effects with quinine and artemether. The antiplasmodial activity and drug interactions were evaluated against the multidrug-resistant W2mef strain of Plasmodium falciparum using the parasite lactate dehydrogenase assay. Three of the four compounds tested were significantly active against W2mef: specicoside (IC(50) = 1.02 ± 0.17 μM), 2β,3β,19α-trihydroxy-urs-12-en-28-oic acid (IC(50) = 1.86 ± 0.15 μM) and atranorin (IC(50) = 1.78 ± 0.18 μM), whereas p-Hydroxy-cinnamic acid showed a weak activity (IC(50) = 12.89 ± 0.87 μM). A slight synergistic effect was observed between atranorin and 2β,3β,19α-trihydroxy-urs-12-en-28-oic acid (Combination index, CI = 0.82) whereas the interaction between specicoside and p-Hydroxy-cinnamic acid were instead antagonistic (CI = 2.67).
CONCLUSIONS:
All the three compounds showed synergistic effects with artemether, unlike the slight antagonistic interactions of atranorin and 2β,3β,19α-trihydroxy-urs-12-en-28-oic acid in combination with quinine. K. africana compounds are therefore likely to serve as leads in the development of new partner drugs in artemether-based combination therapy. | | Nat Prod Commun. 2012 Oct;7(10):1351-2. | | Thymofolinoates A and B, new cinnamic acid derivatives from Euphorbia thymifolia.[Pubmed: 23157007] | | Two new cinnamic acid derivatives, thymofolinoates A (1) and B (2) have been isolated from the chloroform soluble fraction of Euphorbia thymifolia and their structures assigned from 1H and 13C NMR spectra, DEPT and by 2 D COSY, HMQCand H MBC experiments. In addition, p-Hydroxy-cinnamic acid(3), 5-hydroxy-6,7,8,4'-tetramethoxy flavone (4), and 5-hydroxy-3',4',6,7,8-pentamethoxy flavone (5) have also been isolated for the first time from this species. |
|
| In vivo: |
| Mol Med Rep. 2009 Jul-Aug;2(4):641-4. | | The bone anabolic carotenoids p-hydroxycinnamic acid and β-cryptoxanthin antagonize NF-κB activation in MC3T3 preosteoblasts.[Pubmed: 21475879] | The carotene p-hydroxycinnamic acid and the xanthophyll β-cryptoxanthin are members of the carotenoid family of plant-derived pigments, which are endowed with anti-osteoporotic properties in vivo. p-Hydroxycinnamic acid and β-cryptoxanthin have been demonstrated to stimulate osteoblastic bone formation while simultaneously repressing osteoclastic bone resorption in vitro. However, their mechanisms of action remain poorly elucidated. It is well established that the NF-;kgr;B signal transduction pathway plays a critical role in osteoclast differentiation. Moreover, we recently demonstrated that NF-κB activity potently antagonizes osteoblastic differentiation and mineralization in vitro.
METHODS AND RESULTS:
In this study, we used transient transfection assays of a NF-κB luciferase reporter to demonstrate that p-hydroxycinnamic acid and β-cryptoxanthin antagonize NF-κB activation in MC3T3 preosteoblastic cells.
CONCLUSIONS:
The data obtained suggest that NF-κB may be a common molecular target by which several bone active agents, including carotenoids, promote osteoblastic bone formation. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)