| Kinase Assay: |
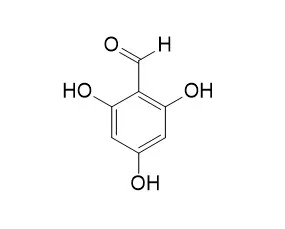
| Medicinal Chemistry Research, 2010, 20(8):1181-1187. | | 2,4,6-Trihydroxybenzaldehyde as a potent antidiabetic agent alleviates postprandial hyperglycemia in normal and diabetic rats.[Reference: WebLink] |
METHODS AND RESULTS:
Five distinct organic compounds with protected and unprotected phenolic hydroxyl groups were screened for their α-glucosidase inhibitory potential. Of these compounds, 2,4,6-Trihydroxybenzaldehyde (THB) showed the strongest noncompetitive α-glucosidase inhibition (IC50 = 4.60 μM) and the most powerful antioxidant activity (DPPH assay) (IC50 = 71.4 μM) in vitro.
CONCLUSIONS:
In in vivo studies, THB significantly reduced blood glucose levels in normal and diabetic rats after glucose load compared to maltose load. |
|
| Cell Research: |
| J Agric Food Chem, 2010, 58(9):5320-5327. | | Gut metabolites of anthocyanins, gallic acid, 3-O-methylgallic acid, and 2,4,6-trihydroxybenzaldehyde, inhibit cell proliferation of Caco-2 cells.[Pubmed: 20373763] | Gut microflora metabolize anthocyanins to phenolic acids and aldehydes. These metabolites may explain the relationship between anthocyanin consumption and reduced incidence of colon cancer.
METHODS AND RESULTS:
Here, all six major metabolites, along with a Cabernet Sauvignon anthocyanin extract, were incubated with Caco-2 cells at concentrations of 0-1000 microM over 72 h to determine effects on cell proliferation and for 24 h to assess cytotoxicity effects and at 140 microM for 24 h to measure induction of apoptosis. These measurements were based on colorimetric methods. Gallic acid and 3-O-methylgallic acid inhibited cell proliferation and lacked cytotoxicity at low concentrations. The aldehyde metabolite and anthocyanin extract also inhibited cell proliferation at low concentrations and had low cytotoxicity at a wide range of concentrations. Of the four substances that effectively reduced cell proliferation, the aldehyde was the best inducer of apoptosis. In addition, these same four treatments degraded quickly in growth media, suggesting the involvement of subsequent oxidation products in the reduction of cell viability.
CONCLUSIONS:
These results indicate that the anthocyanin microfloral metabolites gallic acid, 3-O-methylgallic acid, and 2,4,6-Trihydroxybenzaldehyde reduce cell proliferation in Caco-2 cells more effectively than anthocyanins and may offer protection against colon cancer after their formation in the gut.
|
|
| Animal Research: |
| Environmental Toxicology and Pharmacology, 2015,39(2):962-968. | | 2,4,6-Trihydroxybenzaldehyde, a potential anti-obesity treatment, suppressed adipocyte differentiation in 3T3-L1 cells and fat accumulation induced by high-fat diet in C57BL/6 mice .[Reference: WebLink] |
METHODS AND RESULTS:
In the present study, 2,4,6-Trihydroxybenzaldehyde (THB) was evaluated for inhibitory effects on adipocyte differentiation in 3T3-L1 cells and anti-obesity effects in mice with high-fat diet (HFD)-induced obesity. Lipid accumulation measurement indicated that THB markedly inhibited adipogenesis, and this involved down-regulation of the expression of the adipogenesis-related proteins, CCAAT/enhancer-binding protein α (C/EBPα), peroxisome proliferator-activated receptor γ (PPARγ), fatty acid synthase (FAS) and sterol regulatory element-binding protein-1c (SREBP-1c), in 3T3-L1 pre-adipocyte cells. In a mouse model of HFD-induced obesity, oral administration of THB (5 and 25 mg/kg for 13 weeks) reduced the HFD-induced increase in weight gain. THB administration also reduced serum levels of glucose, triglycerides, and total cholesterol. A reduction in the hypertrophy of white adipose tissue was also observed. Furthermore, THB administration inhibited HFD-induced hepatic steatosis.
CONCLUSIONS:
These results provided evidence that administration of THB alleviated HFD-induced obesity in C57BL/6 mice and revealed the potential of THB as a nutraceutical to help prevent or treat obesity and the associated metabolic disorders. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)