| Cell Research: |
| Autophagy. 2008 Nov;4(8):1009-19. | | Dihydrocapsaicin (DHC), a saturated structural analog of capsaicin, induces autophagy in human cancer cells in a catalase-regulated manner.[Pubmed: 18818525] | Although capsaicin, a pungent component of red pepper, is known to induce apoptosis in several types of cancer cells, the mechanisms underlying capsaicin-induced cytotoxicity are unclear.
METHODS AND RESULTS:
Here, we showed that Dihydrocapsaicin (DHC), an analog of capsaicin, is a potential inducer of autophagy. DHC was more cytotoxic than capsaicin in HCT116, MCF-7 and WI38 cell lines. Capsaicin and DHC did not affect the sub-G(1) apoptotic peak, but induced G(0)/G(1) arrest in HCT116 and MCF-7 cells. DHC caused the artificial autophagosome marker GFP-LC3 to redistribute and upregulated expression of autophagy-related proteins. Blocking of autophagy by 3-methyladenine (3MA) as well as siRNA Atg5 induced a high level of caspase-3 activation. Although pretreatment with zVAD completely inhibited caspase-3 activation by 3MA, it did not prevent cell death. DHC-induced autophagy was enhanced by zVAD pretreatment, as shown by increased accumulation of LC3-II protein. DHC attenuated basal ROS levels through catalase induction; this effect was enhanced by antioxidants, which increased both LC3-II expression and caspase-3 activation. The catalase inhibitor 3-amino-1,2,4-triazole (3AT) abrogated DHC-induced expression of LC3-II, overexpression of the catalase gene increased expression of LC3-II protein, and knockdown decreased it. Additionally, DHC-induced autophagy was independent of p53 status.
CONCLUSIONS:
Collectively, DHC activates autophagy in a p53-independent manner and that may contribute to cytotoxicity of DHC. |
|
| Structure Identification: |
| J Pharm Biomed Anal. 2014 Oct 25;103C:59-66. | | A validated HPLC-FLD method for analysis of intestinal absorption and metabolism of capsaicin and dihydrocapsaicin in the rat.[Pubmed: 25462121] |
METHODS AND RESULTS:
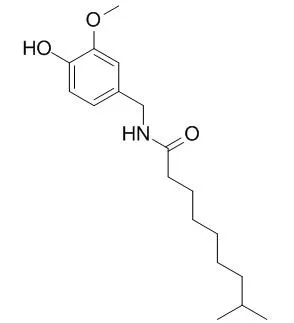
A sensitive and selective reverse-phase high performance liquid chromatographic method with fluorescence detection has been developed for determination of capsaicin (8-methyl-N-vanillyl-(trans)-6-nonenamid) and Dihydrocapsaicin (8-methyl-N-vanillylnonanamide) in samples generated in rat small intestine luminal perfusion experiments. The experiments were designed to study the biotransformation of capsaicinoids in the small intestine in the rat. The chromatographic separation was performed at room temperature on a ZORBAX Eclipse(®) XDB-C8 column using isocratic elution with a mobile phase consisting 0.05M orthophosphoric acid solution and acetonitrile (60:40, v/v; pH 3.0) with a flow rate of 1.5mL/min. Fluorescence detection was performed at excitation and emission wavelengths of 230 and 323nm, respectively. The method was evaluated for a number of validation characteristics (accuracy, repeatability and intermediate precision, limit of detection, limit of quantification and calibration range). The limit of detection (LOD) was 50ng/mL and the limit of quantification (LOQ) was 100ng/mL for both capsaicin and Dihydrocapsaicin reference standards dissolved in blank perfusate. The method was successfully applied for investigation of intestinal absorption of capsaicin and Dihydrocapsaicin while 30μg/mL standardized Capsicum extract - containing capsaicin and Dihydrocapsaicin - was luminally perfused for a 90min period.
CONCLUSIONS:
The structure of the glucuronide metabolites of capsaicin and Dihydrocapsaicin appeared in the perfusate was identified by mass spectrometry. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)