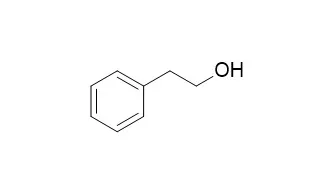
| Journal of Bacteriology, 1967, 93(2):560. |
| Mechanism of action of phenethyl alcohol: breakdown of the cellular permeability barrier.[Reference: WebLink] |
METHODS AND RESULTS:
Phenethyl alcohol (PEA) caused Escherichia coli to take up greatly increased amounts of acriflavine, a compound to which healthy growing cells are impermeable. PEA also caused an increased rate of efflux (leakage) of cellular potassium under conditions which do not greatly alter the influx of potassium via the energy-dependent potassium pump.
CONCLUSIONS:
We therefore propose that the primary effect of PEA is a limited breakdown of the cell membrane. The inhibition of deoxyribonucleic acid synthesis and other cellular functions would then be secondary consequences of the alteration in the membrane structure. |
| Journal of Bacteriology, 1962, 83(4):738. |
| Selective and reversible inhibition of the synthesis of bacterial deoxyribonucleic acid by phenethyl alcohol.[Reference: WebLink] |
METHODS AND RESULTS:
The selective inhibitory effects of Phenethyl alcohol on gram-negative bacteria were confirmed with a variety of species. At a concentration of 0.25%, Phenethyl alcohol was bacteriostatic for gram-negative bacteria; gram-positive cells were unaffected.
Pseudomonas fluorescens required higher concentrations of the compound for inhibition than did the other gram-negative bacteria, and the gram-positive, acid-fast mycobacteria resembled the majority of gram-negative bacteria in sensitivity. In the presence of Phenethyl alcohol, gram-negative cells formed long filaments. There was no net synthesis of deoxyribonucleic acid (DNA) in such cells, whereas protein and ribonucleic acid (RNA) syntheses were unaffected. Upon removal of Phenethyl alcohol, multiplication of the cells immediately ensued, with concomitant DNA synthesis.
CONCLUSIONS:
Yeast extract stimulated both RNA and protein synthesis in Phenethyl alcohol-treated Escherichia coli, but no detectable stimulation of DNA synthesis occurred under these conditions. |
| Journal of Bacteriology, 1972, 109(1):162. |
| Effects of phenethyl alcohol on phospholipid metabolism in Escherichia coli.[Reference: WebLink] |
METHODS AND RESULTS:
The incorporation of labeled precursors into the deoxyribonucleic acid, ribonucleic acid (RNA), proteins, and phospholipids of Escherichia coli cultured in the presence of Phenethyl alcohol (PEA) was determined. PEA inhibited the uptake of labeled uracil to the same extent in cells exhibiting relaxed and stringent control of RNA synthesis. This indicates that PEA does not primarily affect amino acid synthesis or activation. Uptake of labeled acetate into the phospholipid fraction was more sensitive to inhibition by low concentrations of PEA than was the uptake of labeled precursors into the macromolecules. Thymine starvation or the addition of nalidixic acid (10 mug/ml) had no effect on acetate incorporation. Chloramphenicol (25 mug/ml) was a much less effective inhibitor of acetate incorporation than was PEA. The distribution of labeled acetate incorporated into phospholipids was markedly affected by the presence of PEA.
The uptake of acetate into phosphatidylethanolamine and phosphatidylglycerol was inhibited, whereas the uptake of acetate into the cardiolipin fraction was unaffected.
CONCLUSIONS:
Since acetate incorporation into phospholipid was quite sensitive to PEA, we suggest that the PEA-sensitive component required for the initiation of replication may be a phospholipid(s). |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)