| Kinase Assay: |
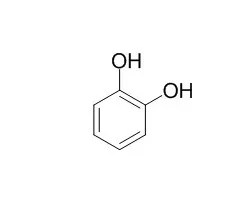
| J Enzyme Inhib Med Chem. 2015 Aug;30(4):586-91. | | Carbonic anhydrase inhibitors: guaiacol and catechol derivatives effectively inhibit certain human carbonic anhydrase isoenzymes (hCA I, II, IX and XII).[Pubmed: 25373500] | | A series of phenolic compounds, including guaiacol, 4-methylguaiacol, 4-propylguaiacol, eugenol, isoeugenol, vanillin, syringaldehyde, 1,2-Benzenediol, 3-methyl catechol, 4-methyl 1,2-Benzenediol and 3-methoxy catechol were investigated for their inhibition of all the catalytically active mammalian isozymes of the Zn(2+)-containing CA (EC 4.2.1.1). All the phenolic compounds effectively inhibited human carbonic anhydrase isoenzymes (hCA I, II, IX and XII), with Kis in the range of 2.20-515.98 μM. The various isozymes showed diverse inhibition profiles. Among the tested phenolic derivatives, compounds 4-methyl 1,2-Benzenediol and 3-methoxy 1,2-Benzenediol showed potent activity as inhibitors of the tumour-associated transmembrane isoforms (hCA IX and XII) in the submicromolar range, with high selectivity. The results obtained from this research may lead to the design of more effective carbonic anhydrase isoenzyme inhibitors (CAIs) based on such phenolic compound scaffolds. |
|
| Animal Research: |
| Electroencephalography & Clinical Neurophysiology, 1973, 35(6):589-601. | | An analysis of the myoclonic jerks produced by 1, 2-dihydroxybenzene in the rat.[Reference: WebLink] |
METHODS AND RESULTS:
In rats deeply anaesthetized with urethane, injection of 1,2 dihydroxybenzene (catechol; 60 mg/kg i.p.) produces a stimulus-sensitive state in which a variety of sensory stimuli, e.g., a brief electrical shock or mechanical tap applied to the periphery, or a binaural click, produce brief (myoclonic) muscular jerks. These jerks have been recorded electromyographically in the forelimb from the biceps and triceps brachii and in the hind limb from gastrocnemius and tibialis anterior. The myoclonic jerks produced in these muscles by electrical or mechanical stimulation of the corresponding paw consisted of three distinct components. The early, first response to electrical stimulation has a short consistent latency (4.3 ± 0.6 msec in the forelimb; 8.1 ± 0.9 msec in the hind limb), large amplitude and high probability of occurence. This first response represents a polysynaptic reflex elicited by stimulation of cutaneous afferents. It is not, however, a simple reflex since it could be recorded in direct antagonists at a similar latency. The first response persisted in the hind limb muscles with both chronic and acute spinal transection at T10-T12. The second response (13.4 ± 1.6 msec in the forelimb; 19.4 ± 3.7 msec in the hind limb) and the third response (40.0 ± 7.3 msec and 51.4 ± 7.3 msec respectively) were both abolished in the hind limb muscles by spinal transection. Records of single motor units in the tibial nerve showed that discharge of a single motoneurone can account for all three components of the reflex jerk. The second, and to a lesser extent, the third response were always reduced in amplitude when the first response was large. Conversely, large second responses were only recorded when the first response was small or absent.
CONCLUSIONS:
Catechol had no effect on the amplitude or latency of the afferent volley recorded at the dorsal root entry zone, but markedly potentiated both mono- and polysynaptic reflexes. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)