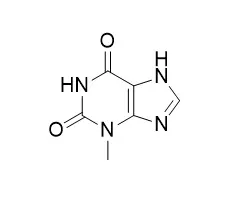
| Structure Identification: |
| Microb Cell Fact. 2015 Dec 21;14:203. | | Direct conversion of theophylline to 3-methylxanthine by metabolically engineered E. coli.[Pubmed: 26691652 ] | Methylxanthines are natural and synthetic compounds found in many foods, drinks, pharmaceuticals, and cosmetics. Aside from caffeine, production of many methylxanthines is currently performed by chemical synthesis. This process utilizes many chemicals, multiple reactions, and different reaction conditions, making it complicated, environmentally dissatisfactory, and expensive, especially for monomethylxanthines and paraxanthine. A microbial platform could provide an economical, environmentally friendly approach to produce these chemicals in large quantities. The recently discovered genes in our laboratory from Pseudomonas putida, ndmA, ndmB, and ndmD, provide an excellent starting point for precisely engineering Escherichia coli with various gene combinations to produce specific high-value paraxanthine and 1-, 3-, and 7-methylxanthines from any of the economical feedstocks including caffeine, theobromine or theophylline. Here, we show the first example of direct conversion of theophylline to 3-Methylxanthine by a metabolically engineered strain of E. coli.
METHODS AND RESULTS:
Here we report the construction of E. coli strains with ndmA and ndmD, capable of producing 3-Methylxanthine from exogenously fed theophylline. The strains were engineered with various dosages of the ndmA and ndmD genes, screened, and the best strain was selected for large-scale conversion of theophylline to 3-Methylxanthine. Strain pDdA grown in super broth was the most efficient strain; 15 mg/mL cells produced 135 mg/L (0.81 mM) 3-Methylxanthine from 1 mM theophylline. An additional 21.6 mg/L (0.13 mM) 1-methylxanthine were also produced, attributed to slight activity of NdmA at the N 3 -position of theophylline. The 1- and 3-Methylxanthine products were separated by preparative chromatography with less than 5% loss during purification and were identical to commercially available standards. Purity of the isolated 3-Methylxanthine was comparable to a commercially available standard, with no contaminant peaks as observed by liquid chromatography-mass spectrophotometry or nuclear magnetic resonance.
CONCLUSIONS:
We were able to biologically produce and separate 100 mg of highly pure 3-Methylxanthine from theophylline (1,3-dimethylxanthine). The N-demethylation reaction was catalyzed by E. coli engineered with N-demethylase genes, ndmA and ndmD. This microbial conversion represents a first step to develop a new biological platform for the production of methylxanthines from economical feedstocks such as caffeine, theobromine, and theophylline. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)