| In vitro: |
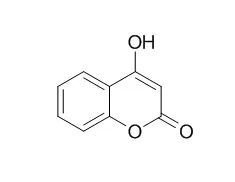
| Bioorg Med Chem. 2011 Nov 1;19(21):6233-8. | | Effect of different C3-aryl substituents on the antioxidant activity of 4-hydroxycoumarin derivatives.[Pubmed: 21964183] | The antioxidant activity of 4-Hydroxycoumarin synthetic derivatives and 4-methylumbelliferone were determined taking 4-Hydroxycoumarin as the reference compound.
METHODS AND RESULTS:
Six 3-aryl-4-Hydroxycoumarin derivatives were synthesized from 4-Hydroxycoumarin as precursor in order to evaluate changes in their antioxidant properties due to C3-aryl substituent nature. Free radical scavenging capacities of these compounds against two different species DPPH(·) and ABTS(·+) and the protecting ability towards the β-carotene-linoleic acid co-oxidation enzymatically induced by lipoxygenase were measured. In addition, the relationship between the activities of these molecules against DPPH radical and the bond dissociation energy of O-H (BDE) calculated using methods of computational chemistry was evaluated. | | Pharm Biol. 2011 Feb;49(2):190-3. | | Larvicidal activity of 4-hydroxycoumarin derivatives against Aedes aegypti.[Pubmed: 21043993] | During our search for new types of coumarin derivatives possessing a larvicidal activity, we investigated the synthesis of 4-Hydroxycoumarin derivatives.
METHODS AND RESULTS:
The structure analyses were conducted by nuclear magnetic resonance (NMR), and mass (MS) spectroscopy revealed that the coumarin derivatives were obtained in good yields, and the eight coumarin derivatives were 3-{1,2,3,4-tetrahydro-3-[4-(4-trifluoromethylbenzyloxy)phenyl}-1-naphthalen-1-on (1), 3-{1,2,3,4-tetrahydro-3-[4-(4-trifluoro methylbenzyloxy)phenyl}-1-naphthalen-1-ol (2), brodifacoum (3), difethialone (4), bromadiolone (5), 4-hydroxy-3-(1,2,3,4-tetrahydronaphthalen-1-yl)-2H-chromen-2-one (coumatetralyl) (6), cis-flocoumafen (7) and trans-flocoumafen (8). The compounds were tested against the F(21) laboratory strain of Aedes aegypti L. Brodifacoum and cis-flocoumafen mediated strong activity with an LC(50) values of 8.23 and 9.34 ppm, respectively.
CONCLUSIONS:
The above indicates that brodifacoum may play a more important role in the toxicity of 4-Hydroxycoumarin derivatives. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)