| Description: |
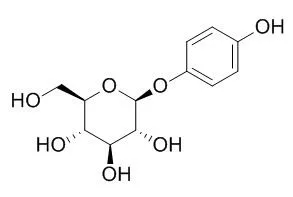
Arbutin is a tyrosinase inhibitor with an IC50 of 1.09 mM, which has gastroprotective, whitening, anti- age, anti-oxidant , anti-inflammatory, a depigmenting effects and UVB/ UVC filter. Arbutin has mutagenicity in mammalian cells after activation by human intestinal bacteria. |
| In vitro: |
| BMC Biochem. 2014 Oct 9;15:23. | | Alleviation effect of arbutin on oxidative stress generated through tyrosinase reaction with L-tyrosine and L-DOPA.[Pubmed: 25297374] |
METHODS AND RESULTS:
Hydroxyl radical that has the highest reactivity among reactive oxygen species (ROS) is generated through L-tyrosine-tyrosinase reaction. Thus, the melanogenesis might induce oxidative stress in the skin. Arbutin (p-hydroxyphenyl-β-D-glucopyranoside), a well-known tyrosinase inhibitor has been widely used for the purpose of skin whitening. The aim of the present study was to examine if Arbutin could suppress the hydroxyl radical generation via tyrosinase reaction with its substrates, L-tyrosine and L-DOPA.
The hydroxyl radical, which was determined by an electron spin resonance-spin trapping technique, was generated by the addition of not only L-tyrosine but L-DOPA to tyrosinase in a concentration dependent manner. Arbutin could inhibit the hydroxyl radical generation in the both reactions.
CONCLUSIONS:
It is presumed that Arbutin could alleviate oxidative stress derived from the melanogenic pathway in the skin in addition to its function as a whitening agent in cosmetics. | | Food Chem Toxicol. 2006 Nov;44(11):1940-7. | | Mutagenicity of arbutin in mammalian cells after activation by human intestinal bacteria.[Pubmed: 16904805 ] | Arbutin (hydroquinone-beta-D-glucopyranoside) is present in various food plants. Its aglycone, hydroquinone, is mutagenic and carcinogenic.
METHODS AND RESULTS:
We investigated whether hydroquinone may be released under conditions encountered in the human gastrointestinal tract. Arbutin was stable in artificial gastric juice. Fecal slurries from nine human subjects completely converted Arbutin (2 mM) into hydroquinone. Four of nine representative human intestinal species investigated, namely Eubacterium ramulus, Enterococcus casseliflavus, Bacteroides distasonis, and Bifidobacterium adolescentis, deglycosylated Arbutin at rates of 21.08, 16.62, 8.43 and 3.59 nmol x min(-1) x (mg protein)(-1), respectively. In contrast, homogenates from small intestinal mucosa and cytosolic fractions from colon mucosa deglycosylated Arbutin at substantially lower rates: 0.50 and 0.09 nmol x min(-1) x (mg protein)(-1), respectively. Arbutin, unlike hydroquinone, did not induce gene mutations in Chinese hamster V79 cells in the absence of an activating system. However, in the presence of cytosolic fractions from E. ramulus or B. distasonis, Arbutin was strongly mutagenic. Cytosolic fraction from Escherichia coli, showing no Arbutin glycosidase activity, was not able to activate Arbutin in this model system.
CONCLUSIONS:
The release of the proximate mutagen hydroquinone from Arbutin by intestinal bacteria in the immediate vicinity of the colon mucosa may pose a potential risk. | | PLoS One . 2017 May 11;12(5):e0177330. | | Action of tyrosinase on alpha and beta-arbutin: A kinetic study[Pubmed: 28493937] | | Abstract
The known derivatives from hydroquinone, α and β-Arbutin, are used as depigmenting agents. In this work, we demonstrate that the oxy form of tyrosinase (oxytyrosinase) hydroxylates α and β-Arbutin in ortho position of the phenolic hydroxyl group, giving rise to a complex formed by met-tyrosinase with the hydroxylated α or β-Arbutin. This complex could evolve in two ways: by oxidizing the originated o-diphenol to o-quinone and deoxy-tyrosinase, or by delivering the o-diphenol and met-tyrosinase to the medium, which would produce the self-activation of the system. Note that the quinones generated in both cases are unstable, so the catalysis cannot be studied quantitatively. However, if 3-methyl-2-benzothiazolinone hydrazone hydrochloride hydrate is used, the o-quinone is attacked, so that it becomes an adduct, which can be oxidized by another molecule of o-quinone, generating o-diphenol in the medium. In this way, the system reaches the steady state and originates a chromophore, which, in turn, has a high absorptivity in the visible spectrum. This reaction allowed us to characterize α and β-Arbutin kinetically as substrates of tyrosinase for the first time, obtaining a Michaelis constant values of 6.5 ± 0.58 mM and 3 ± 0.19 mM, respectively. The data agree with those from docking studies that showed that the enzyme has a higher affinity for β-Arbutin. Moreover, the catalytic constants obtained by the kinetic studies (catalytic constant = 4.43 ± 0.33 s-1 and 3.7 ± 0.29 s-1 for α and β-Arbutin respectively) agree with our forecast based on 13 C NMR considerations. This kinetic characterization of α and β-Arbutin as substrates of tyrosinase should be taken into account to explain possible adverse effects of these compounds. |
|
| In vivo: |
| J Ethnopharmacol. 2012 May 7;141(1):273-81. | | Gastroprotective activities of Turnera diffusa Willd. ex Schult. revisited: Role of arbutin.[Pubmed: 22374081] | Turnera diffusa Willd. ex Schult. has been used for the treatment of several human disorders including peptic ulcer.
The current study is an attempt to evaluate the anti-ulcerogenic activities of Arbutin, a major constituent of Turnera diffusa on two ulcer models. The possible involvement of lipid peroxidation, nitric oxide, IL-6, IL-10, TNF-α and mucus barrier mechanism has been investigated.
METHODS AND RESULTS:
Effects of Arbutin on ulcer index, gastric juice acidity, mucus content and histochemistry, gross and histological gastric lesions, nitric oxide, cytokines levels (IL-6, IL-10 and TNF-α), and thiobarbituric acid reactive substances (TBARS), were evaluated in aspirin or ethanol-induced ulcer in vivo. Acute toxicity of Arbutin was also examined in rodent model. MTT assay was used to assess the cytotoxicity of the compound on normal liver cells (WRL-68).
Pre-treatment with Arbutin or omeprazole protected the gastric mucosa as seen by reduction in ulcer area and mucosal content, reduced or absence of edema, inflammation and leucocytes infiltration on both models. Arbutin significantly (P<0.05) lowered the elevated TBARS level into gasteric homogenate. Arbutin did not produce significant inhibition of NO. This natural compound has modulated the levels of interleukin-6, interleukin-10 and TNF-α. No in vitro or in vivo toxicities for Arbutin were observed.
CONCLUSIONS:
Thus it can be concluded that Turnera diffusa possesses anti-ulcer activity, which could be attributed to lipid peroxidation inhibitory, immuno modulatory and anti-oxidant mechanisms of Arbutin but not to the intervention with nitric oxide inflammation pathway. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)