| In vitro: |
| Cytotechnology. 2017 Aug;69(4):725-739. | | Modulatory effects of Terminalia arjuna against domoic acid induced toxicity in Caco-2 cell line.[Pubmed: 28342004 ] | Domoic acid is a potent marine algal toxin produced by diatomic genus of Pseudo-nitzschia causing amnesic shell fish poisoning. Domoic acid toxicosis mainly involves excitotoxic effects coupled with oxidative stress.
METHODS AND RESULTS:
The present study was aimed to evaluate the protective effects of hydro-alcoholic extract of Terminalia arjuna (TA) against domoic acid induced toxic effects in Caco-2 cell line. It was observed that the toxicity induced by domoic acid in Caco-2 cells was mediated by oxidative insult leading to morphological changes, DNA damage and apoptosis. In our study pre-treatment of the cells with TA (10, 20 and 30 μg/ml) showed significant protection against domoic acid induced morphological, oxidative and apoptotic damages in a dose dependent manner. The effect of phytocompounds present in TA viz., kaempferol and Arjungenin showed significant protection against domoic acid induced toxicity in Caco-2 cell line.
CONCLUSIONS:
Hence, it could be inferred that the protective effect of TA extract against domoic acid induced toxicity could be due to the individual or synergistic effects of kaempferol and argungenin. However, further clinical studies are warranted to consider TA as a natural remedy to prevent amnesic shell fish poisoning. | | Fitoterapia. 2016 Apr;110:89-95. | | Antibacterial and cytotoxic triterpenoids from the roots of Combretum racemosum.[Pubmed: 26946378] | A new pentacyclic triterpenoid glucoside, together with fourteen known compounds, was isolated from the roots of Combretum racemosum. Combretaceae).
METHODS AND RESULTS:
The structure of the new compound was established as 28-O-β-d-glucopyranosyl-2α,3β,21β,23-tetrahydroxyolean-18-en-28-oate (1) on the basis of detailed spectroscopic data including MS, 1D, and 2D NMR. The inhibitory activity of compounds 1-15 against promyelocytic leukemia HL-60 and human erythromyeloblastoid leukemia K562 cell lines was evaluated. Compounds 11 (3-O-β-acetyl-ursolic acid), 14 (betulinic acid), and 15 (quadranoside II) exhibited significant cytotoxicity, with IC50 values of 13 to 50 μM. Among the isolated triterpenes, compounds 1, 3 (Arjungenin), 5 (terminolic acid), and 11 exhibited moderate antibacterial activity against Staphylococcus aureus, Escherichia coli and Enterococcus faecalis (MICs within a range of 64 and 256 μg/mL). | | Zeitschrift Für Naturforschung B, 2005, 60(3):347-350. | | Some chemical constituents of Terminalia glaucescens and their enzymes inhibition activity.[Reference: WebLink] |
METHODS AND RESULTS:
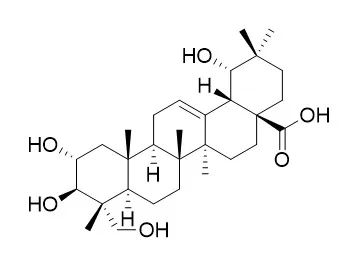
A new triterpenoid, glaucinoic acid (2α, 3β, 19α, 24-tetrahydroxyolean-12-en-30-oic acid) (1) along with several known compounds, arjunic acid (2), Arjungenin (3), sericoside (4), and friedelin (5) were isolated from the stem barks of Terminalia glaucescens.
CONCLUSIONS:
These compounds showed β-glucuronidase inhibitory activity. The structures were identified on the basis of spectroscopic techniques.
|
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)