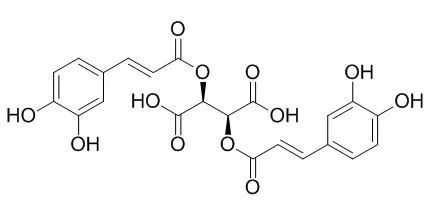
| Description: |
Chicoric acid, a new compound able to enhance insulin release and glucose uptake, it is a new potential antidiabetic agent carrying both insulin sensitizing and insulin-secreting properties. Chicoric acid has antiobesity effects, it can induce apoptosis in 3T3-L1 preadipocytes through ROS-mediated PI3K/Akt and MAPK signaling pathways. L-Chicoric acid has antiviral activity against HIV-1, which has been attributed to the inhibition of HIV-1 integration. |
| Targets: |
TLR | NOS | PARP | MMP(e.g.TIMP) | Bcl-2/Bax | Caspase | p38MAPK | JNK | ERK | HO-1 | COX | Akt | PI3K | HIV | NO | PGE | NF-kB | TNF-α |
| In vitro: |
| J Agric Food Chem. 2013 Feb 20;61(7):1509-20. | | Chicoric acid induces apoptosis in 3T3-L1 preadipocytes through ROS-mediated PI3K/Akt and MAPK signaling pathways.[Pubmed: 23363008] | Chicoric acid has been reported to possess various bioactivities. However, the antiobesity effects of Chicoric acid remain poorly understood.
METHODS AND RESULTS:
In this study, we investigated the effects of Chicoric acid on 3T3-L1 preadipocytes and its molecular mechanisms of apoptosis. Chicoric acid inhibited cell viability and induced apoptosis in 3T3-L1 preadipocytes which was characterized by chromatin condensation and poly ADP-ribose-polymerase (PARP) cleavage. Mitochondrial membrane potential (MMP) loss, Bax/Bcl-2 dysregulation, cytochrome c release, and caspase-3 activation were observed, indicating mitochondria-dependent apoptosis induced by Chicoric acid. Furthermore, PI3K/Akt and MAPK (p38 MAPK, JNK, and ERK1/2) signaling pathways were involved in Chicoric acid-induced apoptosis. The employment of protein kinase inhibitors LY294002, SB203580, SP600125, and U0126 revealed that PI3K/Akt signaling pathway interplayed with MAPK signaling pathways. Moreover, Chicoric acid induced reactive oxygen species (ROS) generation.
CONCLUSIONS:
Pretreatment with the antioxidant N-acetylcysteine (NAC) significantly blocked cell death and changes of Akt and MAPK signalings induced by Chicoric acid. In addition, Chicoric acid down regulated HO-1 and COX-2 via the PI3K/Akt pathway. | | Biochem Biophys Res Commun. 2008 Dec 5;377(1):131-5. | | Chicoric acid, a new compound able to enhance insulin release and glucose uptake.[Pubmed: 18834859 ] | Caffeic acid and chlorogenic acid (CGA), a mono-caffeoyl ester, have been described as potential antidiabetic agents.
METHODS AND RESULTS:
Using in vitro studies, we report the effects of a dicaffeoyl ester, Chicoric acid (CRA) purified from Cichorium intybus, on glucose uptake and insulin secretion. Our results show that CRA and CGA increased glucose uptake in L6 muscular cells, an effect only observed in the presence of stimulating concentrations of insulin. Moreover, we found that both CRA and CGA were able to stimulate insulin secretion from the INS-1E insulin-secreting cell line and rat islets of Langerhans. In the later case, the effect of CRA is only observed in the presence of subnormal glucose levels. Patch clamps studies show that the mechanism of CRA and CGA was different from that of sulfonylureas, as they did not close K(ATP) channels.
CONCLUSIONS:
Chicoric acid is a new potential antidiabetic agent carrying both insulin sensitizing and insulin-secreting properties. |
|
| In vivo: |
| Fitoterapia. 2014 Mar;93:132-41. | | Identification of chicoric acid as a hypoglycemic agent from Ocimum gratissimum leaf extract in a biomonitoring in vivo study.[Pubmed: 24418658] | Ocimum gratissimum L. is popularly used to treat diabetes mellitus. The hypoglycemic activity of this medicinal species has been confirmed by in vivo studies.
METHODS AND RESULTS:
The present study conducted a chemical investigation of a leaf decoction (10% p/v) of O. gratissimum monitored by in vivo hypoglycemic activity assays. Four phenolic substances were identified: L-caftaric acid (1), L-Chicoric acid (2), eugenyl-β-D-glucopyranoside (3) and vicenin-2 (4). The acute hypoglycemic activity of the O. gratissimum decoction fractions Og1-S (300 mg/kg), Og1-A (240 mg/kg) and Og1-B (80 mg/kg) was evaluated intraperitoneally in normal and streptozotocin-induced diabetic mice. They reduced glycemia by 63%, 76% and 60% (in 120 min), respectively, in the diabetic mice. Subfractions of Og1-A were also evaluated under the same conditions: Og1-AS (200 mg/kg) and Og1-AP (40 mg/kg) produced a decrease of only 37% and 39%, respectively. Among the major phenolic substances, only Chicoric acid (2; 3 mg/kg) reduced significantly the glycemic levels of diabetic mice by 53%, 120 min after treatment. This is the first study describing the hypoglycemic activity of Chicoric acid in an animal model of diabetes mellitus. In addition, we suggest that there may be other substances contributing to this activity.
CONCLUSIONS:
Thus, for the first time, a correlation is established between the hypoglycemic activity of O. gratissimum and its chemical composition. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)