| Structure Identification: |
| Zhongguo Zhong Yao Za Zhi. 2015 Feb;40(4):679-85. | | Chemical constituents of Osmanthus fragrans.[Pubmed: 26137690] | By Silica gel, Sephadex LH-20 and other materials for isolation and purification and by physicochemical methods and spectral analysis for structural identification, 32 compounds were isolated and identified from ethyl acetate portion of alcohol extract of the Osmanthus fragrans.
METHODS AND RESULTS:
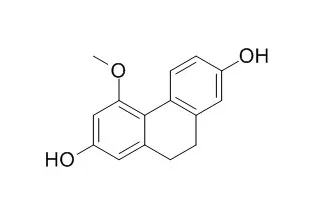
Their structures were identified as boschniakinic acid (1), ursolaldehyde (2), augustic acid (3), arjunolic acid (4), 5-hydroxymethyl-2-furancarboxaldehyde (5), isoscutellarein (6), 6, 7-dihydroxycoumarin (7), 2α-hydroxy-oleanolic acid (8), quercetin-3-0-β-D-glu-copyranoside (9), D-allito (10), 5, 4'-dihydroxy-7- methoxyflavone-3-0-β-D-glucopyranoside (11), 5,7-dihydroxychromone (12), lupeol (13), naringenin (14), acetyloleanolic acid (15), chlorogenic acid (16), kaempferol-3-0-β- D-glucopyranoside (17), oleanolic acid (18), kaempferol-3-0-β-D-galactopyanoside (19), 3', 7-dihydroxy-4'-methoxyisoflavon (20), ergosta-4,6,8 (14), 22-tetraen-3-one (21), p-hydroxycinnamic acid (22), syringaresinol (23), 3,4-dihydroxyacetophenonel (24), β-sitosterol (25), ethyl p-hydroxyphenylacetate (26), benzoic acid (27), caffeic acid (28), Coelonin (29), p-hydorxy-phenylacetic acid (30), p-hydroxyacetophenone (31), and methyl-p-hydroxphenylacetate (32). Except for compounds 2, 4, 5, 8-11, 13, 15, 18, 20, 25, and 27, the rest were isolated from the Osmanthus fragrans for the first time. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)