| Description: |
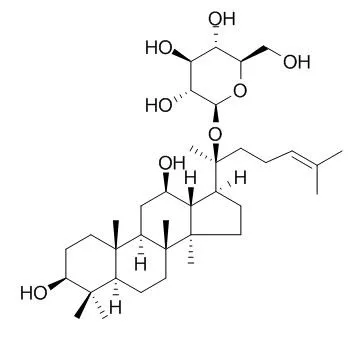
Ginsenoside compound K (C-K) is a metabolite of the protopanaxadiol-type saponins of Panax ginseng C.A. Meyer, has long been used to treat against the development of cancer, inflammation, allergies, and diabetes; C-K acts as a unique HUVEC migration inhibitor by regulating MMP expression, as well as the activity of SPHK1 and its related sphingolipid metabolites. C-K exhibits anti-inflammatory effects by reducing iNOS and COX-2, C-K exhibits an inhibition against the activity of CYP2C9 and CYP2A6 in human liver microsomes with IC50s of 32.0±3.6 μM and 63.6±4.2 μM, respectively. C-K promotes Aβ clearance by enhancing autophagy via the mTOR signaling pathway in primary astrocytes. |
| In vitro: |
| Fitoterapia. 2015 Jan;100:208-20. | | A review of biotransformation and pharmacology of ginsenoside compound K.[Pubmed: 25449425] | | As an intestinal bacterial metabolite of ginseng protopanaxadiol saponins, Ginsenoside Compound K (20-O-beta-d-glucopyranosyl-20(S)-protopanaxadiol, CK) is a major deglycosylated metabolite form of ginsenosides which is absorbed into the systemic circulation. And it has demonstrated such diverse intriguing biological properties as anticarcinogenic, anti-inflammation, antiallergic, anti-diabetic, anti-angiogenesis, anti-aging, neuroprotective and hepatoprotective effects. The present review shall summarize recent studies on various biotransformation and pharmacological activities of CK. | | Planta Med. 2011 Mar;77(5):428-33. | | Ginsenoside compound K attenuates metastatic growth of hepatocellular carcinoma, which is associated with the translocation of nuclear factor-κB p65 and reduction of matrix metalloproteinase-2/9.[Pubmed: 20979019 ] | The intestinal metabolite of ginseng saponin, Ginsenoside Compound K (CK), has various chemopreventive and chemotherapeutic activities, including anti-tumor activity. However, the functional mechanisms through which CK attenuates metastatic growth in hepatocellular carcinoma (HCC) remain unclear.
METHODS AND RESULTS:
Here, using multiple IN VITRO and IN VIVO models, we reported that CK strongly attenuated colony formation, adhesion, and invasion of HCC cells IN VITRO and dramatically inhibited spontaneous HCC metastatic growth IN VIVO. At the molecular level, immunofluorescence and Western blotting analysis confirmed that inhibition of metastatic growth of HCC induced by CK treatment caused a time-dependent decrease in nuclear NF- κB p65 and a concomitant increase in cytosolic NF- κB p65, indicating that CK suppressed the activation of the NF- κB pathway. Meanwhile, our study showed that the inhibition of matrix metalloproteinase2/9 (MMP2/9) expression caused by CK treatment was associated with NF- κB p65 nuclear export.
CONCLUSIONS:
Taken together, our results not only revealed that NF- κB p65 nuclear export and the reduction of MMP2/9 expression were associated with the metastatic inhibition induced by CK, but also suggested that CK may become a potential cytotoxic drug in the prevention and treatment of HCC.
| | J Ginseng Res . 2017 Oct;41(4):435-443. | | Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases[Pubmed: 29021688] | | Panax ginseng is one of the most universally used herbal medicines in Asian and Western countries. Most of the biological activities of ginseng are derived from its main constituents, ginsenosides. Interestingly, a number of studies have reported that ginsenosides and their metabolites/derivatives-including ginsenoside (G)-Rb1, compound K, G-Rb2, G-Rd, G-Re, G-Rg1, G-Rg3, G-Rg5, G-Rh1, G-Rh2, and G-Rp1-exert anti-inflammatory activities in inflammatory responses by suppressing the production of proinflammatory cytokines and regulating the activities of inflammatory signaling pathways, such as nuclear factor-κB and activator protein-1. This review discusses recent studies regarding molecular mechanisms by which ginsenosides play critical roles in inflammatory responses and diseases, and provides evidence showing their potential to prevent and treat inflammatory diseases. | | Acta Pharmacol Sin . 2014 May;35(5):599-612. | | Ginsenoside compound K suppresses the abnormal activation of T lymphocytes in mice with collagen-induced arthritis[Pubmed: 24727939] | | Aim: To investigate the anti-arthritis and immunomodulatory activities of Ginsenoside Compound K (C-K) in mice with collagen-induced arthritis (CIA).
Methods: DBA/1 mice with CIA were treated with C-K (28, 56 or 112 mg·kg(-1)·d(-1), ig) or the positive control methotrexate (2 mg/kg, ig, every 3 d) for 34 d. Splenic T and B lymphocytes were positively isolated using anti-CD3-coated magnetic beads or a pan B cell isolation kit. T lymphocyte subsets, and CD28, T cell receptor (TCR), cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and programmed death-1 (PD-1) expression in purified splenic T lymphocytes were analyzed using flow cytometry, Western blotting and laser confocal microscopy.
Results: C-K treatment significantly ameliorated the pathologic manifestations of CIA mice, remarkably inhibited T lymphocyte proliferation, and marginally inhibited the proliferation of B lymphocytes. C-K treatment significantly suppressed TNF-α and anti-CII antibody levels, and increased IFN-γ level in the joints of CIA mice, but did not alter IL-4 production. Treatment of CIA mice with C-K significantly decreased the percentages of activated T cells, co-stimulatory molecule-expressing T cells and effector memory T cells, and increased the frequencies of naive T cells and regulatory T cells. Furthermore, C-K treatment significantly decreased the expression of CD28 and TCR, whereas it increased the expression of CTLA-4 and PD-1 on T lymphocytes of CIA mice. Methotrexate treatment exerted comparable effects in all these experiments. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)