| Description: |
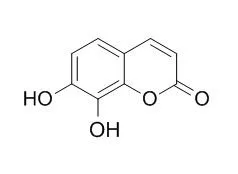
Daphnetin is a dihydroxycoumarin that is being used in China for the treatment of coagulation disorders, is a protein kinase inhibitor, inhibits EGFR, PKA and PKC with IC50 of 7.67 μM, 9.33 μM and 25.01 μM, respectively, also known to exhibit antimalarial, anti-rheumatoid arthritis, anti-proliferative, anti-inflammatory and anti-oxidant activities. it is also a chelator and an antioxidant. Daphnetin can enhance immunological functions of B lymphocytes, the expression of IL-12 in B lymphocytes can be up-regulated by daphnetin through natural immunity approach.
|
| In vitro: |
| Eur J Pharmacol. 2011 Oct 1;668(1-2):35-41. | | Differential effects of esculetin and daphnetin on in vitro cell proliferation and in vivo estrogenicity.[Pubmed: 21741969] | Esculetin (6,7-dihydroxycoumarin) and Daphnetin (7,8-dihydroxycoumarin) are secondary metabolites of plants used in folk medicine. These compounds have showed great antiproliferative activity in several tumor cell lines and have been proposed as potential anticancer agents. However, the estrogenic potential of these two compounds has to date not been reported.
METHODS AND RESULTS:
The present study compared esculetin and Daphnetin on the inhibition of cell proliferation and cell cycle progression of the MCF-7 estrogen-responsive human carcinoma cell line. In vivo and in vitro estrogenic activity for both compounds was also evaluated. Esculetin inhibited cell proliferation after 72 h exposure (IC50=193 ± 6.6 μM), while Daphnetin evidenced inhibiting effects starting at 24-h exposure (72 h, IC50=73 ± 4.1 μM). Both effects showed changes in cyclin D1 gene expression. In non-estrogenic conditions (E-screening assay), esculetin produced biphasic response on proliferation of the MCF-7 cells; at 10(-8)-10(-6)M, concentrations induced proliferative effects as EC50=4.07 × 10(-9)M (E(2)=2.91 × 10(-12)M); at higher concentrations (10(-5)-10(-4)M), cell proliferation was inhibited. Relative proliferative effect at E(2) was 52% (E(2)=100), relative proliferative potency was 0.072 (E(2)=100). Additionally, esculetin tested in vivo showed estrogenic effects at 50-100mg/kg doses; relative uterotrophic effect at E(2) was 37%, with relative uterotrophic potency registered at 0.003.
In contrast, Daphnetin did not induce estrogenic effects in vitro or with in vivo models. The low estrogenic activity of esculetin could prove useful in postmenopausal therapy but not as a safe antitumor agent in estrogen-dependent tumors.
CONCLUSIONS:
Daphnetin-based antiproliferative selectivity with MCF-7 cells showed that Daphnetin is a promising antitumoral agent also acting on estrogen dependent tumors. | | Am J Trop Med Hyg. 1992 Jan;46(1):15-20. | | Daphnetin: a novel antimalarial agent with in vitro and in vivo activity.[Pubmed: 1311154] | | Daphnetin is a dihydroxycoumarin that is being used in China for the treatment of coagulation disorders. It is also a chelator and an antioxidant. In vitro, Daphnetin causes a 50% inhibition (IC50) of 3H-hypoxanthine incorporation by Plasmodium falciparum at concentrations between 25 and 40 microM. Several related compounds, such as scopoletin, 2, 3-dihydroxybenzoic acid and 3, 4-dihydroxybenzoic acid show no inhibitory activity. The antimalarial activity of Daphnetin is inhibited by the addition of iron. Daphnetin does not appear to be an oxidant drug, since it does not spontaneously generate superoxide in vitro. However, it does alkylate bovine serum albumin when incubated in the presence of iron. In vivo, Daphnetin significantly prolongs survival of P. yoelli-infected mice. | | Free Radic Biol Med . 2017 May;106:38-52. | | Daphnetin-mediated Nrf2 antioxidant signaling pathways ameliorate tert-butyl hydroperoxide (t-BHP)-induced mitochondrial dysfunction and cell death[Pubmed: 28188924] | | Abstract
Daphnetin (Daph), a natural coumarin derivative isolated from plants of the Genus Daphne, possesses abundant biological activities, such as anti-inflammatory, antioxidant and anticancer properties. In the present study, we focused on investigating the protective effect of Daph against tert-butyl hydroperoxide (t-BHP)-induced oxidative damage, mitochondrial dysfunction and the involvement of underlying molecular mechanisms. Our findings indicated that Daph effectively inhibited t-BHP-stimulated cytotoxicity, cell apoptosis, and mitochondrial dysfunction, which are associated with suppressed reactive oxygen species (ROS) generation, decreased malondialdehyde (MDA) formation, increased superoxide dismutase (SOD) levels and glutathione (GSH)/GSSG (oxidized GSH) ratio. Further investigation indicated that Daph significantly suppressed cytochrome c release and NLRP3 inflammasome activation and modulated apoptosis-related protein Bcl-2, Bax, and caspase-3 expression. Moreover, Daph dramatically induced the expression of the glutamate-cysteine ligase modifier (GCLM) subunit and the glutamate-cysteine ligase catalytic (GCLC) subunit, heme oxygenase-1 (HO-1), and NAD (P) H: quinone oxidoreductase (NQO1), which is largely dependent on upregulating the nuclear factor-erythroid 2-related factor 2 (Nrf2) nuclear translocation, reducing the Keap1 protein expression, and strengthening the antioxidant response element (ARE) promoter activity. Additionally, Daph remarkably activated a c-Jun NH2-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK) phosphorylation, but ERK and JNK inhibitor pretreatment exhibited an evident decrease of the level of Daph-enhanced Nrf2 nuclear translocation. Furthermore, Daph exposure suppressed t-BHP-induced cytotoxicity and ROS overproduction, which are mostly blocked in Nrf2 knockout RAW 264.7 cells and peritoneal macrophages. Accordingly, Daph exhibited protective roles against t-BHP-triggered oxidative damage and mitochondrial dysfunction by the upregulation of Nrf2 antioxidant signaling pathways, which may be involved in the activation of JNK and ERK.
Keywords: Daphnetin; Mitochondrial dysfunction; Nrf2; Oxidative damage; ROS. |
|
| In vivo: |
| J Agric Food Chem. 2014 Dec 24;62(51):12315-25. | | Anti-inflammatory and protective properties of daphnetin in endotoxin-induced lung injury.[Pubmed: 25419854] | Uncontrolled inflammatory responses cause tissue injury and severe immunopathology. Pharmacological interference of intracellular pro-inflammatory signaling may confer a therapeutic benefit under these conditions.
Daphnetin, a natural coumarin derivative, has been used to treat inflammatory diseases including bronchitis. However, the protective effect of Daphnetin in inflammatory airway disorders has yet to be determined, and the molecular basis for its anti-inflammatory properties is unknown.
METHODS AND RESULTS:
This paper shows that Daphnetin treatment conferred substantial protection from endotoxin-induced acute lung injury (ALI), in parallel with reductions in the production of inflammatory mediators, symptoms of airway response, and infiltration of inflammatory cells. Further studies indicate that activation of macrophage and human alveolar epithelial cells in response to lipopolysaccharide (LPS) was remarkably suppressed by Daphnetin, which was related to the down-regulation of NF-κB-dependent signaling events. Importantly, this study demonstrates that TNF-α-induced protein 3 (TNFAIP3), also known as A20, was significantly induced by Daphnetin, which appeared to be largely responsible for the down-regulation of NF-κB activity through modulation of nondegradative TRAF6 ubiquitination. Accordingly, the deletion of TNFAIP3 in primary macrophages reversed Daphnetin-elicited inhibition of immune response, and the beneficial effect of Daphnetin in the pathogenesis of ALI was, partially at least, abrogated by TNFAIP3 knockdown.
CONCLUSIONS:
These findings demonstrate the anti-inflammatory and protective functions of Daphnetin in endotoxin-induced lung inflammation and injury and also reveal the key mechanism underlying its action in vitro as well as in vivo. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)