| Description: |
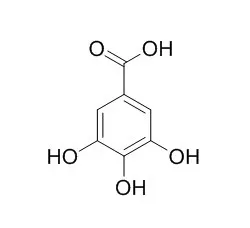
Gallic acid, is a histone acetyltransferase inhibitor and a potent inhibitor of brush border sucrase and other disaccharidases, which has powerful antioxidant, anti-tumor, and anti-tyrosinase activities. It can potentially interfere with the digestive functions of the intestine. It can efficiently block neuronal cell death by downregulating the expression of cytokines and the in vivo levels of NF-κB acetylation, is a possible therapeutic approach for alleviating the inflammatory progression of Alzheimer disease. |
| Targets: |
NF-kB | Beta Amyloid | Tyrosinase |
| In vitro: |
| Food Chem., 2002, 79(3):307-13. | | Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid[Reference: WebLink] | The antioxidant and pro-oxidant properties of ascorbic acid (AA) and Gallic acid (GA) were investigated.
METHODS AND RESULTS:
AA and GA, at a concentration of 1.65 mM, accelerate the oxidation of deoxyribose induced by Fe3+–EDTAJH2O2. The reducing power of these two compounds increased upon increasing the concentration. AA and GA showed no chelating ability toward iron (II). At a concentration of 4.17 mM, AA and GA exhibited 42.1 and 43.9% scavenging effects on DPPH radicals, respectively. They exhibited 60% scavenging effects on hydrogen peroxide at a concentration of 4.17 mM. No toxicity was found in AA and GA toward human lymphocytes. AA, at 0.82 mM, and GA, at 0.6 mM, exhibited the maximal DNA damage, the means of tail DNA% were 14.8 and 28.8%, respectively. When AA and GA were mixed with H2O2, they exhibited a slight inhibitory effect on DNA damage induced by H2O2 on pre-incubating both the compounds with human lymphocytes for 30 min before exposure to H2O2.
CONCLUSIONS:
The antioxidant activities of AA and GA at a higher concentration were mainly due to the scavenging of hydrogen peroxide in this system. The pro-oxidant mechanism for AA and GA acid is most likely due to the strong reducing power and weak metalchelating ability. | | Biol Pharm Bull. 2007 Jun;30(6):1052-5. | | Antimelanogenic and antioxidant properties of gallic acid.[Pubmed: 17541153] | To find novel skin-whitening agents, the melanogenesis inhibitory action of Gallic acid (GA) was investigated.
METHODS AND RESULTS:
In this current study, the effects of GA on mushroom tyrosinase, tyrosinase inhibitory activity, and melanin content were assessed in B16 melanoma cells (B16 cells). Results indicated that GA has a strong antityrosinase activity (IC50=3.59x10(-6) M). Furthermore, data on murine tyrosinase activity and melanin biosynthesis revealed that GA effectively suppressed murine tyrosinase action and the amount of melanin. To investigate the relation between GA's inhibition of melanogenesis and antioxidant activity, the effect of GA on reactive species (RS) generation and the reduced glutathione (GSH)/oxidized glutathione (GSSG) ratio in were determined in B16 cells. Results indicated that GA effectively down-regulated the RS generation and enhanced the GSH/GSSG ratio.
CONCLUSIONS:
Based on these results, I propose that GA exerts antimelanogenic activity coupled with antioxidant properties by suppressing RS generation and maintaining a higher GSH/GSSG ratio. | | Springerplus . 2012 Dec;1(1):58. | | COX-2 structural analysis and docking studies with gallic acid structural analogues[Pubmed: 23483789] | | Emblica officinalis is an ayurvedic herbal plant. The compounds isolated from this plant have good inhibitory effects against cyclooxygenase-2 (COX-2), among them Gallic acid (GA) has the highest inhibitory effect. COX-2 (1.14.99.1) is an oxidoreductase having a role in prostaglandin biosynthesis, inflammatory responses and in cardiovascular events. COX-2 has gained special focus on research since past few decades. The sequence and structural studies reveals Mus musculus COX-2 shares the common conserved sequence and structural pattern with human COX-2. Molecular modeling and docking analysis with Gallic acid and their structural analogues showed that 2-[(2E,4E)-hexa-2,4-dienyl]-3,4,5-trihydroxybenzoic acid, (3,4,5-trihydroxybenzoyl) 3,4,5-trihydroxybenzoate and 3-hydroxy-4-sulfooxybenzoic acid are more interactive and binding strongly than Gallic acid at active site. Hence these three compounds should be considered as strong inhibitors for COX-2. | | Oxid Med Cell Longev . 2015;2015:434052. | | Plant Natural Products Calycosin and Gallic Acid Synergistically Attenuate Neutrophil Infiltration and Subsequent Injury in Isoproterenol-Induced Myocardial Infarction: A Possible Role for Leukotriene B4 12-Hydroxydehydrogenase?[Pubmed: 26265982] | | Leukotriene B4 12-hydroxydehydrogenase (LTB4DH) catalyzes the oxidation of proinflammatory LTB4 into less bioactive 12-oxo-LTB4. We recently discovered that LTB4DH was induced by two different natural products in combination. We previously isolated Gallic acid from Radix Paeoniae through a bioactivity-guided fractionation procedure. The purpose of this study is to test the hypothesis that LTB4DH inducers may suppress neutrophil-mediated inflammation in myocardial infarction. We first isolated the active compound(s) from another plant, Radix Astragali, by the similar strategy. By evaluating LTB4DH induction, we identified calycosin and formononetin from Radix Astragali by HPLC-ESI-MS technique. We confirmed that Gallic acid and commercial calycosin or formononetin could synergistically induce LTB4DH expression in HepG2 cells and human neutrophils. Moreover, calycosin and Gallic acid attenuated the effects of LTB4 on the survival and chemotaxis of neutrophil cell culture. We further demonstrated that calycosin and Gallic acid synergistically suppressed neutrophil infiltration and protected cardiac integrity in the isoproterenol-induced mice model of myocardial infarction. Calycosin and Gallic acid dramatically suppressed isoproterenol-induced increase in myeloperoxidase (MPO) activity and malondialdehyde (MDA) level. Collectively, our results suggest that LTB4DH inducers (i.e., calycosin and Gallic acid) may be a novel combined therapy for the treatment of neutrophil-mediated myocardial injury. |
|
| In vivo: |
| Nut. Res., 2007, 27(4):230-5. | | Gallic acid inhibits brush border disaccharidases in mammalian intestine.[Reference: WebLink] | Intestinal epithelium constitutes the primary target tissue for interaction with dietary micronutrients. The objective of this study was to determine if Gallic acid, a polyphenol that is an important constituent of various edible plant-based foods, affects brush border disaccharidases in mammalian intestine.
METHODS AND RESULTS:
In this investigation, we found that 0.05 to 0.6 mmol/L Gallic acid inhibited sucrase activity by 34% to 86%. Optimum enzyme inhibition was observed at 0.4-mmol/L Gallic acid concentration, which was 82% in the rat, 83% in LACA/L mice, 50% in BALB/c mice, and 28% in rabbit intestine. The observed enzyme inhibition was reversible in rat intestines. Gallic acid also depressed the activities of maltase (42%), trehalase (45%), and lactase (13%) in the rat. Inhibition of sucrase activity by Gallic acid was mainly between pH 4.8 to 7.2, whereas at alkaline pH (7.7-8.5), Gallic acid stimulated enzyme activity by 20% to 30% in both rat and rabbit intestines. Kinetic analysis revealed that Gallic acid was a fully competitive inhibitor of rat sucrase at pH 5.5 and 6.8. The effect of Gallic acid together with various -SH group–reacting reagents showed that the observed inhibition was additive in nature. Similar results were obtained in the presence of 0.4 mmol/L Gallic acid and 4 mmol/L harmaline, a plant alkaloid.
CONCLUSIONS:
These findings suggest that Gallic acid is a potent inhibitor of brush border sucrase and other disaccharidases and thus could potentially interfere with the digestive functions of the intestine. | | Anticancer Drugs. 2001 Nov;12(10):847-52. | | Anti-tumor effect of gallic acid on LL-2 lung cancer cells transplanted in mice.[Pubmed: 11707653] | We previously reported that Gallic acid (3,4,5-trihydroxybenzoic acid), a naturally occurring plant phenol, can induce apoptosis in four kinds of human lung cancer cell lines in vitro. The present study further investigated the in vivo anti-tumor effects of orally administered Gallic acid.
METHODS AND RESULTS:
Gallic acid reduced cell viability of LL-2 mouse lung cancer cells in vitro dose dependently, with a 50% inhibitory concentration (IC50) value of around 200 microM. C57Black mice were transplanted with LL-2 cells, and administered Gallic acid (1 mg/ml in drinking water, ad libitum) and/or cisplatin (4 mg/kg i.p. injection, once a week). The average weight of the transplanted tumors, obtained at 29 days after transplantation, in the mice of control, Gallic acid-treated cisplatin-treated and cisplatin plus Gallic acid-treated groups was 4.02, 3.65, 3.19 and 1.72 g, respectively. The average tumor weight of the mice treated with cisplatin combined with Gallic acid was significantly smaller than that of the control group (p<0.05). The amount of apoptotic cells in the tumor tissues of mice treated with Gallic acid and/or cisplatin was significantly higher than those of the control mice. Combination of Gallic acid and cisplatin increased the tumor cell apoptosis compared with the treatment with cisplatin alone.
CONCLUSIONS:
The present findings suggest that the combination of Gallic acid with an anti-cancer drug, including cisplatin, may be an effective protocol for lung cancer therapy. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)