| Description: |
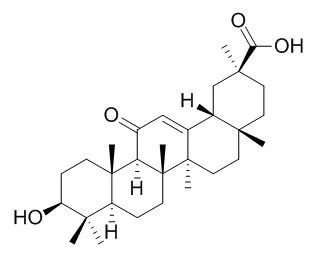
Glycyrrhetinic acid, an AChE activator, has anti-inflammatory,and antileukaemic activities. It is a potent inducer of mitochondrial permeability transition and can trigger the pro-apoptotic pathway, it has a low but definite affinity for mineralocorticoid receptors and thus appears to have a direct mineralocorticoid action. |
| Targets: |
PERK | CDK | E2F‑1 | AChR |
| In vitro: |
| Int J Oncol. 2015 Mar;46(3):981-8. | | Glycyrrhetinic acid induces G1‑phase cell cycle arrest in human non‑small cell lung cancer cells through endoplasmic reticulum stress pathway.[Pubmed: 25573651] | Glycyrrhetinic acid (GA) is a natural compound extracted from liquorice, which is often used in traditional Chinese medicine.
METHODS AND RESULTS:
The purpose of the present study was to investigate the antitumor effect of GA in human non‑small cell lung cancer (NSCLC), and its underlying mechanisms in vitro. We have shown that GA suppressed the proliferation of A549 and NCI‑H460 cells. Flow cytometric analysis showed that GA arrested cell cycle in G0/G1 phase without inducing apoptosis. Western blot analysis indicated that GA mediated G1‑phase cell cycle arrest by upregulation of cyclin‑dependent kinase inhibitors (CKIs) (p18, p16, p27 and p21) and inhibition of cyclins (cyclin‑D1, ‑D3 and ‑E) and cyclin‑dependent kinases (CDKs) (CDK4, 6 and 2). GA also maintained pRb phosphorylation status, and inhibited E2F transcription factor 1 (E2F‑1) in both cell lines. GA upregulated the unfolded proteins, Bip, PERK and ERP72. Accumulation of unfolded proteins in the endoplasmic reticulum (ER) triggered the unfolded protein response (UPR), which could be the mechanism by which GA inhibited cell proliferation in NSCLC cells. GA then coordinated the induction of ER chaperones, which decreased protein synthesis and induced cell cycle arrest in the G1 phase.
CONCLUSIONS:
This study provides experimental evidence to support the development of GA as a chemotherapeutic agent for NSCLC. | | Biochem Pharmacol. 2003 Dec 15;66(12):2375-9. | | Glycyrrhetinic acid-induced permeability transition in rat liver mitochondria.[Pubmed: 14637195] | | Glycyrrhetinic acid, a hydrolysis product of one of the main constituents of licorice, the triterpene glycoside of glycyrrhizic acid, when added to rat liver mitochondria at micromolar concentrations induces swelling, loss of membrane potential, pyridine nucleotide oxidation, and release of cytochrome c and apoptosis inducing factor. These changes are Ca(2+) dependent and are prevented by cyclosporin A, bongkrekic acid, and N-ethylmaleimide. All these observations indicate that Glycyrrhetinic acid is a potent inducer of mitochondrial permeability transition and can trigger the pro-apoptotic pathway. |
|
| In vivo: |
| J. Pharm. Pharmacol., 2011, 10(1):613-20. | | The antiinflammatory activity of glycyrrhetinic acid and derivatives.[Reference: WebLink] | | The anti-inflammatory activities of different fractions of Glycyrrhetinic acid or glycyrrhetic acid and some of its derivatives have been assessed in laboratory animals. Some, but not all, preparations have been found to be active using four established methods for testing anti-inflammatory drugs. The findings provide a scientific basis for the clinical use of these compounds in inflammatory diseases, and may explain the discrepancies in the early clinical trials with this drug. | | Evid Based Complement Alternat Med . 2017;2017:3470320. | | Protective Effect of 18 β-Glycyrrhetinic Acid against Triptolide-Induced Hepatotoxicity in Rats[Pubmed: 28572827] | | Abstract
Triptolide (TP) is the major active component of Tripterygium wilfordii Hook F (TWHF) and possesses multiple pharmacological effects. However, hepatotoxicity of TP which is one of the toxic properties slows its progression in clinical application. 18β-Glycyrrhetinic acid (GA) is the main bioactive ingredient of Licorice (Glycyrrhiza glabra L.), a herbal medicine famous for its detoxification. This study aims to investigate whether GA possesses protective effect against TP-induced hepatotoxicity in rats. TP interference markedly elevated serum levels of ALT, AST, and ALP, caused evident liver histopathological changes, and elevated hepatic TNF-α, IL-6, IL-1β, and IFN-γ as well as nuclear translocation of NF-κB. TP also significantly elevated liver MDA and declined hepatic activities of SOD, CAT, and GSH-Px. Assay of TUNEL and apoptosis proteins (Bax, Bcl-2, and active caspase-3) showed that TP induced severe hepatocellular apoptosis. In contrast, low-dose GA (50 mg/kg) significantly reversed TP-induced changes above. However, high-dose GA (100 mg/kg) had no such effect. Overall, these findings indicated that low-dose GA but not high-dose GA exhibited a protective effect against TP-induced hepatotoxicity in rats by anti-inflammation, antioxidation, and antiapoptosis, which suggests that the doses of GA/Licorice should be carefully considered when used together with TWHF or TWHF preparations. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)