| Molecules. 2016 May 24;21(6). |
| Bioactive Constituents of Zanthoxylum rhetsa Bark and Its Cytotoxic Potential against B16-F10 Melanoma Cancer and Normal Human Dermal Fibroblast (HDF) Cell Lines.[Pubmed: 27231889 ] |
Zanthoxylum rhetsa is an aromatic tree, known vernacularly as "Indian Prickly Ash". It has been predominantly used by Indian tribes for the treatment of many infirmities like diabetes, inflammation, rheumatism, toothache and diarrhea.
METHODS AND RESULTS:
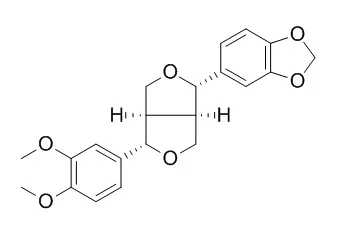
In this study, we identified major volatile constituents present in different solvent fractions of Z. rhetsa bark using GC-MS analysis and isolated two tetrahydrofuran lignans (yangambin and Kobusin), a berberine alkaloid (columbamine) and a triterpenoid (lupeol) from the bioactive chloroform fraction. The solvent fractions and purified compounds were tested for their cytotoxic potential against human dermal fibroblasts (HDF) and mouse melanoma (B16-F10) cells, using the MTT assay. All the solvent fractions and purified compounds were found to be non-cytotoxic to HDF cells. However, the chloroform fraction and Kobusin exhibited cytotoxic effect against B16-F10 melanoma cells.
CONCLUSIONS:
The presence of bioactive lignans and alkaloids were suggested to be responsible for the cytotoxic property of Z. rhetsa bark against B16-F10 cells. |
| J Ethnopharmacol. 2011 Nov 18;138(2):637-40. |
| Furfuran lignans and a flavone from Artemisia gorgonum Webb and their in vitro activity against Plasmodium falciparum.[Pubmed: 21982788 ] |
METHODS AND RESULTS:
The chemical composition of the aerial parts of the Cape Verdean endemic shrub Artemisia gorgonum Webb (Asteraceae) was careful investigated, which led to the isolation and identification of six known furfuran lignans: eudesmin (1), magnolin (2), epimagnolin A (3), aschantin (4), Kobusin (5), sesamin (6) and a flavone: artemetin (7). Compounds 1-7 were evaluated in vitro for their cytotoxicity in a screening panel consisting of various mammalian tumor cell lines, for their antimalarial activity against chloroquine-resistant Plasmodium falciparum (FcB1 strain) and for their cytotoxicity against murine normal cells (CFU-GM). While no promising cytotoxicity against human tumor cells were noticed, marginal potency and selectivity was found for compounds 1-5 against murine colon 38. Besides, compounds 2-7 showed mild antiplasmodial activities, 6 and 7 being the most active compounds (IC(50) 3.37 and 3.50 μg/ml respectively) without noticeable toxicity on mammalian normal cells.
CONCLUSIONS:
This is the first report of antiplasmodial activity for furfuran lignans and the first isolation of 1-7 from Artemisia gorgonum. |
| Phytother Res. 2010 May;24(5):748-53. |
| Suppression of inducible nitric oxide synthase expression by furfuran lignans from flower buds of Magnolia fargesii in BV-2 microglial cells.[Pubmed: 19943243] |
Activated microglia produces diverse neurotoxic factors such as nitric oxide (NO) and tumor necrosis factor-alpha that serve as apoptotic inducers resulting in various neurodegenerative diseases. The inhibition of microglia-derived NO production by inducible nitric oxide synthase (iNOS) has been reported to be beneficial in retarding neurodegenerative disorders.
METHODS AND RESULTS:
Three active lignans have been isolated from the flower buds of Magnolia fargesii by the bioassay-guided fractionation using lipopolysaccharide (LPS)-activated BV-2 microglial cell culture system. The structures of them were identified as Kobusin (1), aschantin (2) and fargesin (3) by the analyses of spectroscopic data. They inhibited the production of NO by activated microglia. Their IC(50) values were 21.8 +/- 3.7, 14.8 +/- 2.5 and 10.4 +/- 2.8 microg/mL, respectively. They suppressed LPS-induced NF-kappaB activation and the expression of iNOS protein and mRNA. Furthermore, they showed scavenging activity of neurotoxic peroxynitrite that can be produced by NO and superoxide anion.
CONCLUSIONS:
These results imply that lignans from Magnolia fargesii might be beneficial for the treatment of neuro-inflammatory diseases through the inhibition of iNOS expression and peroxynitrite scavenging potential. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)