| In vitro: |
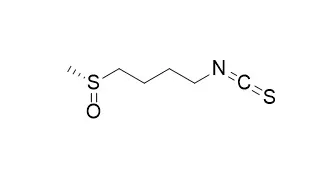
| Int J Cancer . 2011 Jun 15;128(12):2775-82. | | The natural chemopreventive phytochemical R-sulforaphane is a far more potent inducer of the carcinogen-detoxifying enzyme systems in rat liver and lung than the S-isomer[Pubmed: 20726001] | | The chemopreventive activity of the phytochemical sulforaphane, (-)1-isothiocyanato-4R-(methylsulfinyl)-butane, present in cruciferous vegetables in substantial amounts in the form of glucosinolate, was demonstrated in animal models of cancer using the racemate, despite the fact that humans are exposed only to the R-enantiomer through the diet. Since a principal mechanism of the chemopreventive activity of sulforaphane is modulation of the carcinogen-metabolising enzyme systems, a study was conducted in precision-cut rat liver and lung slices, and in FAO cells comparing the ability of R- and S-sulforaphane to modulate these enzyme systems. R-sulforaphane elevated hepatic glutathione S-transferase and quinone reductase whereas the S-enantiomer had no effect; moreover, the R-enantiomer was more effective in up-regulating GSTα, GSTμ and quinone reductase protein levels. In the lung, both enantiomers increased the same enzyme activities with the R-enantiomer being more potent; in addition, the R-enantiomer was more effective in up-regulating GSTα and quinone reductase protein levels. Both isomers increased glutathione levels in both tissues, with R-sulforaphane being more potent. Finally, R-sulforaphane was the more effective of the two isomers in up-regulating CYP1A1/1B1 apoprotein levels in both liver and lung, and CYP1A2 in the liver. Similarly, in FAO cells the R-enantiomer was far more effective in up-regulating quinone reductase and glutathione S-transferase activities and protein levels compared with the S-isomer. These studies demonstrate clearly the superiority of R-sulforaphane, when compared with the S-enantiomer, in stimulating detoxification enzymes, and raises the possibility that the animal studies that employed the racemate may have underestimated the chemopreventive activity of this isothiocyanate. | | Nutrients . 2021 Feb 12;13(2):602. | | Examination of Novel Immunomodulatory Effects of L-Sulforaphane[Pubmed: 33673203] | | The dietary isothiocyanate L-Sulforaphane (LSF), derived from cruciferous vegetables, is reported to have several beneficial biological properties, including anti-inflammatory and immunomodulatory effects. However, there is limited data on how LSF modulates these effects in human immune cells. The present study was designed to investigate the immunomodulatory effects of LSF (10 μM and 50 μM) on peripheral blood mononuclear cell (PBMC) populations and cytokine secretion in healthy adult volunteers (n = 14), in the presence or absence of bacterial (lipopolysaccharide) and viral (imiquimod) toll-like receptor (TLRs) stimulations. Here, we found that LSF reduced pro-inflammatory cytokines interleukin (IL)-6, IL-1β, and chemokines monocyte chemoattractant protein (MCP)-1 irrespective of TLR stimulations. This result was associated with LSF significantly reducing the proportion of natural killer (NK) cells and monocytes while increasing the proportions of dendritic cells (DCs), T cells and B cells. We found a novel effect of LSF in relation to reducing cluster of differentiation (CD) 14 monocytes while simultaneously increasing monocyte-derived DCs (moDCs: lineage-Human Leukocyte Antigen-DR isotype (HLA-DR)CD11b++low-high CD11chigh). LSF was also shown to induce a 3.9-fold increase in the antioxidant response element (ARE) activity in a human monocyte cell line (THP-1). Our results provide important insights into the immunomodulatory effects of LSF, showing in human PBMCs an ability to drive differentiation of monocytes towards an immature monocyte-derived dendritic cell phenotype with potentially important biological functions. These findings provide insights into the potential role of LSF as a novel immunomodulatory drug candidate and supports the need for further preclinical and phase I clinical studies. | | Curr Mol Pharmacol . 2018;11(3):237-253. | | L-Sulforaphane Confers Protection Against Oxidative Stress in an In Vitro Model of Age-Related Macular Degeneration[Pubmed: 29376497] | | Background: In age-related macular degeneration, oxidative damage and abnormal neovascularization in the retina are caused by the upregulation of vascular endothelium growth factor and reduced expression of Glutathione-S-transferase genes. Current treatments are only palliative. Compounds from cruciferous vegetables (e.g. L-Sulforaphane) have been found to restore normal gene expression levels in diseases including cancer via the activity of histone deacetylases and DNA methyltransferases, thus retarding disease progression.
Objective: To examine L-Sulforaphane as a potential treatment to ameliorate aberrant levels of gene expression and metabolites observed in age-related macular degeneration.
Method: The in vitro oxidative stress model of AMD was based on the exposure of Adult Retinal Pigment Epithelium-19 cell line to 200μM hydrogen peroxide. The effects of L-Sulforaphane on cell proliferation were determined by MTS assay. The role of GSTM1, VEGFA, DNMT1 and HDAC6 genes in modulating these effects was investigated using quantitative real-time polymerase chain reaction. The metabolic profiling of L-Sulforaphane-treated cells via gas-chromatography massspectrometry was established. Significant differences between control and treatment groups were validated using one-way ANOVA, student t-test and post-hoc Bonferroni statistical tests (p<0.05).
Results: L-Sulforaphane induced a dose-dependent increase in cell proliferation in the presence of hydrogen peroxide by upregulating Glutathione-S-Transferase μ1 gene expression. Metabolic profiling revealed that L-Sulforaphane increased levels of 2-monopalmitoglycerol, 9, 12, 15,-(Z-Z-Z)- Octadecatrienoic acid, 2-[Bis(trimethylsilyl)amino]ethyl bis(trimethylsilyl)-phosphate and nonanoic acid but decreased β-alanine levels in the absence or presence of hydrogen peroxide, respectively.
Conclusion: This study supports the use of L-Sulforaphane to promote regeneration of retinal cells under oxidative stress conditions. | | Blood Coagul Fibrinolysis . 2013 Jul;24(5):498-504. | | Antiplatelet activity of L-sulforaphane by regulation of platelet activation factors, glycoprotein IIb/IIIa and thromboxane A2[Pubmed: 23412354] | | L-Sulforaphane was identified as an anticarcinogen that could produce quinine reductase and a phase II detoxification enzyme. In recent decades, multi-effects of L-Sulforaphane may have been investigated, but, to the authors' knowledge, the antiplatelet activation of L-Sulforaphane has not been studied yet.In this study, 2 μg/ml of collagen, 50 μg/ml of ADP and 5 μg/ml of thrombin were used for platelet aggregations with or without L-Sulforaphane. L-Sulforaphane inhibited the platelet aggregation dose-dependently. Among these platelet activators, collagen was most inhibited by L-Sulforaphane, which markedly decreased collagen-induced glycoprotein IIb/IIIa activation and thromboxane A2 (TxA2) formation in vitro. L-Sulforaphane also reduced the collagen and epinephrine-induced pulmonary embolism, but did not affect prothrombin time (PT) in vivo. This finding demonstrated that L-Sulforaphane inhibited the platelet activation through an intrinsic pathway.L-Sulforaphane had a beneficial effect on various pathophysiological pathways of the collagen-induced platelet aggregation and thrombus formation as a selective inhibition of cyclooxygenase and glycoprotein IIb/IIIa antagonist. Thus, we recommend L-Sulforaphane as a potential antithrombotic drug. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)