| In vitro: |
| J Agric Food Chem. 2006 Mar 22;54(6):2359-64. | | Photosensitizing effect of riboflavin, lumiflavin, and lumichrome on the generation of volatiles in soy milk.[Pubmed: 16536619] |
METHODS AND RESULTS:
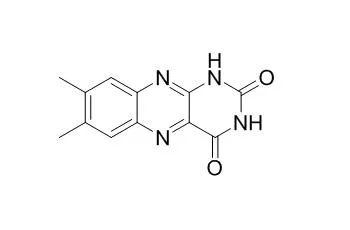
Lumichrome and lumiflavin were formed from riboflavin under light. pH had a significant influence on the formation of Lumichrome and lumiflavin from riboflavin. Lumichrome was the only major product from riboflavin under neutral or acidic pH values. Lumiflavin was also formed from riboflavin in basic pH. The maximum concentration of lumiflavin from 100 microM riboflavin at pH 8.5 was 30.9 microM, and it was reached after 2 h of exposure at 1500 lux. The maximum concentration of Lumichrome formed from 100 microM riboflavin at pH 4.5, 6.5, or 8.5 was 79.9, 58.7, and 73.1 microM, respectively, after 8, 6, or 2 h of light exposure. The formation of Lumichrome and lumiflavin from riboflavin was due to the type I mechanism of the riboflavin photosensitized reaction. Singlet oxygen was also involved in the photosensitized degradation of lumiflavin and Lumichrome. The reaction rates of riboflavin, lumiflavin, and Lumichrome with singlet oxygen were 9.66 x 10(8), 8.58 x 10(8), and 8.21 x 10(8) M(-1) s(-1), respectively. The headspace oxygen depletion and headspace volatile formation were significant in soy milk containing Lumichrome or lumiflavin under light (p < 0.05) and were insignificant (p > 0.05) in the dark.
CONCLUSIONS:
Ascorbic acid could inhibit the total volatile changes of soy milk under light. Soy milk should be protected from light to prevent the photodegradation of riboflavin and the oxidation of soy milk. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)