| In vitro: |
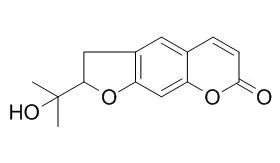
| Molecules. 2014 Oct 22;19(10):16959-75. | | Antioxidant, 5-lipoxygenase inhibitory and cytotoxic activities of compounds isolated from the Ferula lutea flowers.[Pubmed: 25340301] | A phytochemical investigation of the Ferula lutea (Poir.) Maire flowers has led to the isolation of a new compound, (E)-5-ethylidenefuran-2(5H)-one-5-O-β-d-glucopyranoside (1), designated ferunide, 4-hydroxy-3-methylbut-2-enoic acid (2), reported for the first time as a natural product, together with nine known compounds, verbenone-5-O-β-d-glucopyranoside (3), 5-O-caffeoylquinic acid (4), methyl caffeate (5), methyl 3,5-O-dicaffeoylquinate (6), 3,5-O-dicaffeoylquinic acid (7), isorhamnetin-3-O-α-l-rhamnopyranosyl(1→6)-β-d-glucopyranoside, narcissin (8), (-)-Marmesin (9), isoimperatorin (10) and 2,3,6-trimethylbenzaldehyde (11).
METHODS AND RESULTS:
Compounds 3-10 were identified for the first time in Ferula genus. Their structures were elucidated by spectroscopic methods, including 1D and 2D NMR experiments, mass spectroscopy and X-ray diffraction analysis (compound 2), as well as by comparison with literature data. The antioxidant, anti-inflammatory and cytotoxic activities of isolated compounds were evaluated. Results showed that compound 7 exhibited the highest antioxidant activity with IC50 values of 18 ± 0.5 μmol/L and 19.7 ± 0.7 μmol/L by DPPH radical and ABTS radical cation, respectively. The compound 6 exhibited the highest anti-inflammatory activity with an IC50 value of 5.3 ± 0.1 μmol/L against 5-lipoxygenase. In addition, compound 5 was found to be the most cytotoxic, with IC50 values of 22.5 ± 2.4 μmol/L, 17.8 ± 1.1 μmol/L and 25 ± 1.1 μmol/L against the HCT-116, IGROV-1 and OVCAR-3 cell lines, respectively. |
|
| In vivo: |
| J Pharm Pharmacol. 2012 Jun;64(6):888-96. | | Hepatoprotective activity of Feronia limonia root.[Pubmed: 22571268] | The aim of this study was to evaluate the hepatoprotective potential of a methanolic extract and of Marmesin isolated from the root bark of Feronia limonia.
METHODS AND RESULTS:
Activity levels of aspartate aminotransaminase (AST) and alanine aminotransaminase (ALT), cell viability and cell death were evaluated in HepG2 cells (human liver hepatoma cells) treated with CCl₄ in the presence or absence of F. limonia extract or Marmesin. Plasma activity levels of AST, ALT, bilirubin, alkaline phosphatase, protein, hepatic antioxidants, lipid peroxidation and histopathological evaluations were carried out in rats treated with CCl₄ alone or co-supplemented with F. limonia extract or Marmesin in a dose-dependent manner. In-vitro co-supplementation of F. limonia methanolic extract or Marmesin significantly minimized alteration in levels of AST and ALT and improved cell viability. Oral administration of F. limonia methanolic extract or Marmesin significantly prevented CCl₄-induced elevation in the plasma markers of hepatic damage and hepatic lipid peroxidation and a decrease in hepatic antioxidants. In-vivo hepatoprotective potential of F. limonia methanolic extract and Marmesin was evident from the minimal alterations in the histoarchitecture of liver.
CONCLUSIONS:
This has been the first scientific report on the hepatoprotective potential of F. limonia root bark methanolic extract and Marmesin. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)